Immune checkpoint inhibitors (ICIs) are a significant breakthrough in cancer therapy. The continuous development of new ICIs and the discovery of biomarkers have considerably improved the therapeutic efficacy of these agents.
Cancer is among the most challenging global health problems, with its occurrence increasing over the years. Cancer immunotherapy recently arose as a highly effective method for cancer treatment.1
Immunotherapy exploits the body’s immune system for the prevention, management, and eradication of cancer, unlike conventional therapy.2
There have been notable cancer immunotherapies established over years of research and development, including cancer vaccines, immune checkpoint inhibitors (ICIs), targeted antibodies, oncolytic virotherapy, remodeling tumor microenvironment (TME), and adoptive cell therapy.3
Immune checkpoints are a co-stimulatory and inhibitory signal to regulate the antigen recognition of T cell receptors (TCR) in the immune response process. They can inhibit immune system overreactions and help to maintain immune homeostasis during antiviral or antimicrobial immune responses.
However, cancer cells can imitate ligands of immune checkpoints to avoid immune surveillance.4
Using ICIs can interrupt the communication between immune checkpoints and their ligands, relieving the immune function suppression and subsequently reactivating the immune cells, allowing them to exert antitumor effects.5
This article presents an overview of the application of ICIs in cancer treatment.
FDA-approved ICIs
The first ICI that was approved for cancer treatment by the FDA was Ipilimumab, a CTLA-4 inhibitor. This was approved in 2011.
Later, PD-1/PD-L1 inhibitors displayed significant antitumor effects in multiple clinical trials and were FDA-approved for many malignancies, such as non-small-cell lung carcinoma, renal cell carcinoma, and melanoma.6
Widely expressed on activated T cells, CTLA-4 and its interaction with receptors on antigen cells terminates the immune responses. CTLA-4 inhibitors trigger T-cell activation and proliferation, which leads to the destruction of tumor cells, as shown in Figure 1.7
PD-1 is an inhibitory receptor that can be found on natural killer (NK) cells, activated T cells, and monocytes. PD-1 ligand 1 (PD-L1) is a transmembrane protein that is expressed on the surface of tumor cells. The interaction between PD-1 and PD-L1 prevents T-cell migration and proliferation, enabling tumor immune evasion.
PD-1/PD-L1 inhibitors block the binding between PD-1 and PD-L1, subsequently reactivating T-cell responses against tumors and realizing antitumor effects.8 The FDA has approved the monoclonal antibodies Pembrolizumab, Nivolumab, and Cemiplimab as PD-1 inhibitors.
Three PD-L1 inhibitors have been approved for use in several solid tumors, including melanoma, MC, NSCLC, and HNSCC (as shown in Figure 1). These inhibitors are namely Avelumab, Atezolizumab, and Durvalumab.
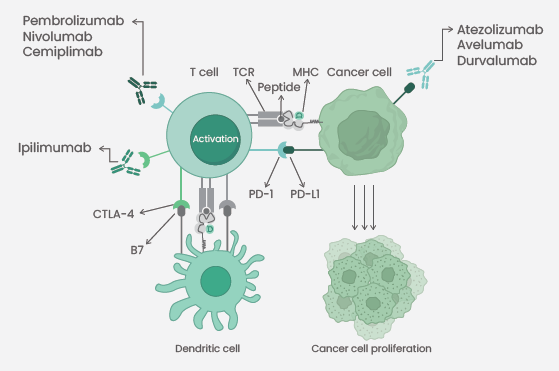
Figure 1. Immune checkpoint inhibitors approved by FDA. Pembrolizumab, Nivolumab, and Cemiplimatb as anti-PD-1 antibodies, Ipilimumab as an anti-CTLA-4 antibody, as well as Atezolizumab, Avelumab, and Durvalumab as anti-PD-L1 antibodies 7. Image Credit: Sino Biological Inc.
To aid combination therapy research, Sino Biological has produced a variety of high-quality proteins, neutralizing antibodies, blocking antibodies, ELISA Kits, and more, as displayed in Figure 2.
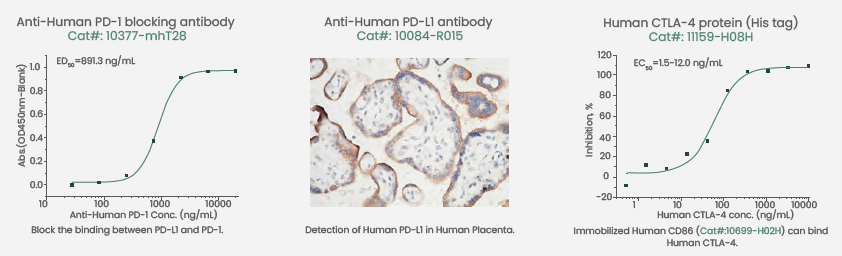
Figure 2. Featured products of high-quality PD-1, PD-L1, and CTLA-4. Image Credit: Sino Biological Inc.
Emerging ICIs
Recently, there has been rapid progress in the development and approval of ICIs for the treatment of various types of cancer.
While PD-1/PD-L1 and CTLA-4 inhibitors are effective, the overall therapeutic efficacy of current ICIs continues to be limited. This is a result of the complex nature of the tumor microenvironment and the diverse characteristics of tumors. Additionally, some ICI therapies are also linked with adverse effects.
Numerous novel immune checkpoints have continually emerged to improve the efficacy of immunotherapy, including TIM-3, VISTA, CD47, LAG-3, CD96, IDO-1, TIGIT, B7-H3, and CD155.
TIGIT
As a member of the immunoglobulin superfamily, TIGIT is mostly expressed on the surface of CD4+ T, CD8+ T, NK, and regulatory T (Treg) cells. It has three ligands, namely CD112, CD113, and CD155.
Structurally, TIGIT is comprised of a type 1 transmembrane domain, an extracellular immunoglobulin (Ig) variable domain, and a cytoplasmic tail with two inhibitory motifs: an Ig tail-tyrosine-like motif and an ITIM.
TIGIT inhibitors hold the potential to improve the antitumor effects, primarily when employed together with PD-1/PD-L1 inhibitors.
VISTA
VISTA is a protein-coding gene that has certain homology with PD-L1 and PD-L2. It is highly expressed in both myeloid suppressor cells and immune cells. VISTA also has high expression levels in a variety of human cancer types, including kidney, lung, and colorectal cancers.
V-Set and the immunoglobulin structural domain containing 3 (VSIG-3) serve as ligands for VISTA, inhibiting the production of cytokines and chemokines.
CD47
CD47 is a protein that is expressed in body cells but is overexpressed in different hematological tumor cells and solid tumors. It serves a central function in modulating tumor immunity through interactions with the signal regulatory protein α (SIRPα). This effectively impedes macrophages from clearing tumor cells.
Presently, the drug research and development for CD47 has largely focused on targeting SIRP α, CD47, and SIRP α-Fc fusion protein of the CD47 molecule within tumor cells.
TIM-3
Encoded by HAVCR2, TIM-3 is a transmembrane protein that is widely expressed in a range of immune cells. TIM-3 interacts with four different ligands, including carcinoembryonic antigen cell adhesion molecule 1 (Ceacam-1), HMGB1, galectin-9, and phosphatidyl serine (PtdSer).
TIM-3 was initially found to be a receptor expressed on IFN-γ-producing CD4+ Th1 and CD8+ T cytotoxicity 1 (Tc1) T cells. However, recent studies have demonstrated that it holds immune evasion abilities like CTLA-4 and PD-1.
Despite many preclinical and clinical studies indicating the potential of TIM-3 as an immune checkpoint, its wide expression across different cell types causes concerns regarding possible serious adverse effects related to systemic antibody applications.
As a result of this, the development of antibody drugs that can selectively target TIM-3 is vital.
Featured products
Sino Biological supports the research of ICIs by offering high-quality immune checkpoint proteins with validated bioactivity, high purity, and broad coverage of tags and species (as displayed in Figure 3).
In addition to this, the company offers ELISA kits, ELISA pair sets for cell-based functional assay and high-quality cytokine proteins.
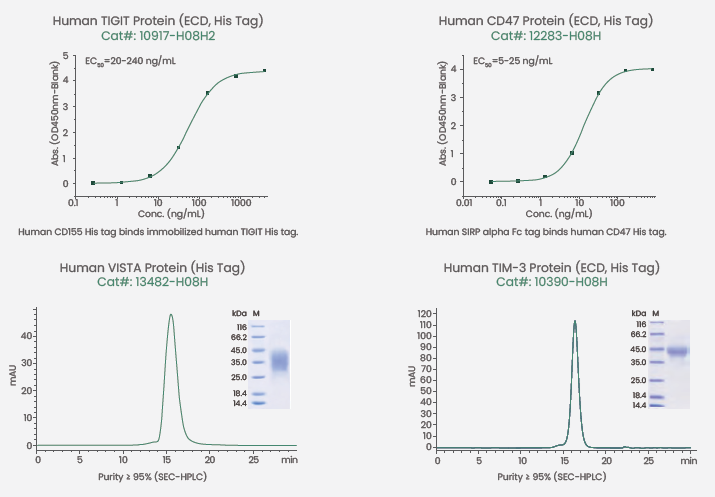
Figure 3. Featured proteins of high-quality TIGIT, VD47, VISTA and TIM-3. Image Credit: Sino Biological Inc.
Combination therapy using ICIs
The combination of multiple ICIs is currently a significant focus of research in tumor immunotherapy. The combination therapy of ICIs with precision and multi-pathway targeting presents unique advantages in addressing drug resistance and improving the specific recognition and destruction of tumor cells by immune cells.
For example, the combination of a CTLA-4 inhibitor with a PD-1 inhibitor has been shown to provide extended survival benefits in lung cancer patients. Combining TIM-3 and PD-1 inhibitors in the treatment of NSCLC helps to offset any resistance to PD-1 inhibitors.
Additionally, the combination of a LAG-3 inhibitor with a CTLA-4 inhibitor can induce immune tolerance through the co-suppression of signaling pathways.9
Predictive biomarkers for ICIs
Treating cancer with immune checkpoint therapy is frequently hindered by low response rates and immune-related adverse events in some cancer patients. As a result, the development of sets of biomarkers for the prediction of immune-related adverse events and immune checkpoint blockade is vital.
Predictive biomarkers can provide insights into a patient’s outcome prior to treatment, assisting in the decision to use either monotherapy or combination therapy. This facilitates the application of precise and tailored tumor immunotherapy.
Currently, PD-L1 and dMMR/MSI-H are approved biomarkers for clinical indication of the efficacy of PD-1/PD-L1 inhibitors, while other potential predictive biomarkers require more clinical study evidence.10
Summary
The introduction of tumor immunotherapy has enabled significant change in the treatment of cancer.
Established ICIs like CTLA-4 and PD-1/PD-L1 inhibitors, as well as emerging ICIs such as VISTA, CD47, TIGIT, and TIM-3 inhibitors, and other inhibitors have found extensive use across a spectrum of cancer types, including melanoma, non-small-cell-lung-cancer, advanced cervical cancer, and hepatocellular carcinoma.
There is also a growing number of clinical studies that are studying immune-combination therapies, such as the combination of different ICIs, pairing ICIs with chemotherapy, radiotherapy, and targeted therapy.
However, it must be noted that the biomarkers currently used for predicting the efficacy of tumor immunotherapy, including TMB, PD-L1 expression levels, and MSI-H, present limitations regarding the accurate prediction of the efficacy of ICIs and therapeutic outcomes.
This means that there is a pressing need to discover more accurate predictive biomarkers for the efficacy of ICIs in the future.
References
- Liu C, Yang M, Zhang D, Chen M, Zhu D. Clinical cancer immunotherapy: Current progress and prospects. Front Immunol. doi:10.3389/fimmu.2022.961805
- Peterson C, Denlinger N, Yang Y. Recent Advances and Challenges in Cancer Immunotherapy. Cancers (Basel). 2022;14(16):3972. doi:10.3390/cancers14163972
- Meng X, Lei Y, Zhang X, et al. Cancer immunotherapy: Classification, therapeutic mechanisms, and nanomaterial-based synergistic therapy. Applied Materials Today, 2021, 24: 101149. doi: 10.1016/j.apmt.2021.101149
- Cai X, Zhan H, Ye Y, et al. Current Progress and Future Perspectives of Immune Checkpoint in Cancer and Infectious Diseases. Front Genet. 2021;12:785153. doi:10.3389/fgene.2021.785153
- Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19(4):254-267. doi:10.1038/s41571-022-00600-w
- Naimi A, Mohammed RN, Raji A, et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun Signal. 2022;20(1):44. doi:10.1186/s12964-022-00854-y
- Shiravand Y, Khodadadi F, Kashani S M A, et al. Immune checkpoint inhibitors in cancer therapy. Current Oncology, 2022, 29(5): 3044-3060. doi:10.3390/curroncol29050247
- Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727-742.
- Vafaei S, Zekiy AO, Khanamir RA, et al. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022;22(1):2. doi:10.1186/s12935-021-02407-8
- Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1-11. doi:10.1038/s12276-018-0191-1
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.