Among humans, the heart is the first organ that develops in the embryo, with vessels emerging in the third week and blood being circulated after thirty days. This is essential for the maintenance of perfusion of the developing fetus. The adult vascular system, which comprises venules, veins, arteries, and arterioles, is rich in receptors that transduce a broad array of extracellular signals. This is crucial for the maintenance of homeostasis.
Blood vessels, particularly the small arterioles, make a key contribution to the establishment of peripheral resistance. In response to endogenous stimuli (which includes the listed chemical messengers), dilation or contraction of a high percentage of vessels in the body will impact on aggregate peripheral resistance and blood pressure. The dysfunction of the vascular endothelium contributes to the balance being shifted from vasodilatation to vasoconstriction. This causes hypertension and is an important initiator of diseases, including stroke, atherosclerosis, and heart failure.
Cardiovascular disease continues to be a significant contributor to morbidity and mortality and accounts for around 30% of total global mortality. This is in spite of the general implementation of drugs such as angiotensin AT1 receptor antagonists and ß-blockers, targeting key G-protein-coupled receptor (GPCR) systems. This indicates that additional targets are yet to be detected. These could potentially be derived from emergent transmitter systems or the remaining orphan receptors.
Vascular Endothelium, Perivascular Nerves, and Smooth Muscle
The vascular endothelium comprises a singular layer of epithelial cells that line all blood vessels in the body and thus occurs in all organs receiving blood supply. It embodies a very large surface area, as well as a mass that is comparable to alternative endocrine glands. The endothelium is no longer believed to be a simple barrier between smooth muscle and blood, but a source of numerous locally acting vasoactive compounds, including nitric oxide (NO) and endothelin-1 (ET-1). Moreover, vascular reactivity is regulated by the release of transmitters from nerves traveling with blood vessels to effectively every organ in the body, as well as hormones that circulate in the plasma.
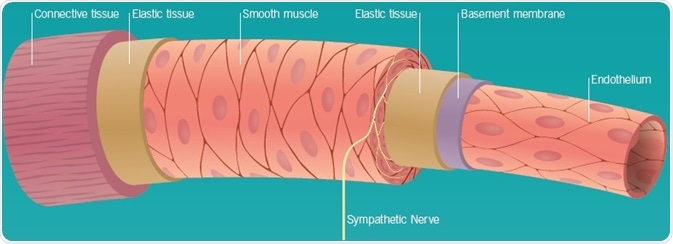
Vascular smooth muscle constitutes the majority of the cell types located in the vessel wall. They exhibit exceptional plasticity as they undergo rapid phenotypic alterations in response to physiological, pathophysiological or mechanical changes in the local environment. The consequent transformation from contractile to a de-differentiated, proliferating phenotype helps to cause the vascular wall to be remodeled. GPCRs regulate several of these alterations.
GPCRs
There is seven shared transmembrane spanning domains among GPCRs. Vertebrate receptors can be split into five major families that are categorized by protein sequence similarity and phylogenetic analysis. These are: Rhodopsin Family (Class A); Secretin Family (Class B); Glutamate Family (Class C); Frizzled Family; and Adhesion Family [1].
GPCRs regulating vascular reactivity are primarily included in the first two of these families.
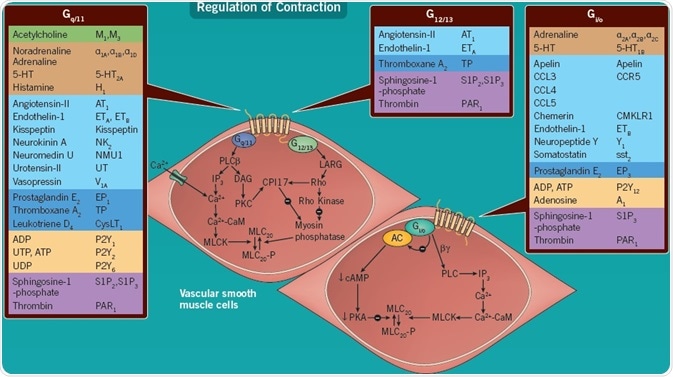
The objective of this poster is to draw attention to those GPCRs which, upon being activated by their cognate ligands, might behave in one of three ways in blood vessels: as indirectly acting vasodilators, or directly acting dilators and constrictors.
The schematic demonstrated herein offers a summary of a generalized blood vessel and its primary functional vasoactive responses to the activation of specified GPCRs. However, there are some key caveats: the expression of receptors with their related G-proteins and consequent functional responses may differ among species and divergent vascular beds, as well as between central and peripheral or arterial and venous vessels.
Additionally, GPCRs can be up- or down-regulated with the disease, dramatically altering the balance between dilation and constriction. For instance, receptor densities are increased in thromboxane TP and atherosclerosis ghrelin, with a down-regulation of angiotensin AT1 receptors in the medial smooth muscle layer of the human coronary artery[2]. Endogenous agonists comprise those which interact with established receptors as well as those which have undergone pairing with previously designated orphan receptors[3,4], where a role in the modulation of vascular reactivity is coming to the fore.
Class A
The superfamily of seven-transmembrane spanning GPCRs belonging to Class A is larger and more diverse than any other family of membrane-spanning proteins. Receptors in Class A constitute the principle target for around one-third of today's available drugs.
In the most recently published list from the IUPHAR/BPS Guide to Pharmacology,[1] 197 non-sensory receptors in Class A are identified as interacting with established transmitters or chemical messengers, including a significant number of vasoactive compounds.
After completion of 99% of the human genome, bioinformatics has been administered to establish most, perhaps even all, of the remaining genes which potentially encode still unliganded ‘orphan’ receptors in this family. As well as the established receptors in Class A where the ligand is known, an additional eighty-nine orphan GPCRs have been suggested to occur within this group[1]. These orphan receptors are still expressed in cell lines and continue to undergo pairing with ligands by screening libraries of extant or new compounds isolated from tissue[3,4]. There are a number of emergent, novel transmitters, and several, including apelin, kisspeptin, and chemerin[5,6,7], exhibit potent activity in the human cardiovascular system. Activation of Class A receptors can engender vasoconstriction or vasodilatation either directly or via the release of endothelium-derived relaxing factors.
| Emerging Vasoconstrictors |
Emerging Vasodilators |
| Agonist |
Receptor |
Agonist |
Receptor |
| CC Chemokines |
CCR5 |
Apelin |
Apelin |
| Chemerin |
CMKLR1 |
Ghrelin |
Ghrelin |
| Kisspeptin |
Kisspeptin |
Nociceptin |
NOP |
| Sphingosine-1-phosphate |
SIP1-3 |
Relaxin |
RXFP1 |
Class B
The secondary category of GPCRs, Class B[8], includes fifteen receptors. Two of these constitute dimers with receptor accessory proteins [receptor activity modifying proteins (RAMPs)] to generate further distinct functional receptor complexes. Up until now, the majority of this family's members undergo activation by thirty to fifty amino acid peptides. These primarily operate in the vasculature as directly acting vasodilators such as the established transmitters, vasoactive intestinal peptide (VIP), calcitonin gene-related peptide (CGRP) and adrenomedullin, in addition to emerging systems, including the urocortin.
Class C
Curiously, the 15 Class C metabotropic receptors which undergo activation by ligands, such as glutamate and GABA, can be localized presynaptically to perivascular nerves, although these are not broadly expressed in the vascular endothelium or smooth muscle.
| Emerging Vasodilators |
| Agonist |
Receptor |
| Urocortin I, II, III |
CRF2 |
Frizzled Family
NC-IUPHAR has identified an additional eleven GPCRs belonging to an unrelated category of the frizzled and smoothened family[1]. Frizzled receptors undergo activation by a family of cysteine-rich glycosylated Wnt protein ligands. Receptors are broadly expressed in the vascular system (in addition to other organs) and have been seen to contribute to remodelings such as cell proliferation, differentiation, and apoptosis, although a contribution to vascular reactivity has not been identified.
Adhesion Family
Some of the 33 receptors, designated orphans, in this family might contribute to the development of the cardiovascular system or angiogenesis, although a contribution to vascular reactivity has not been identified.
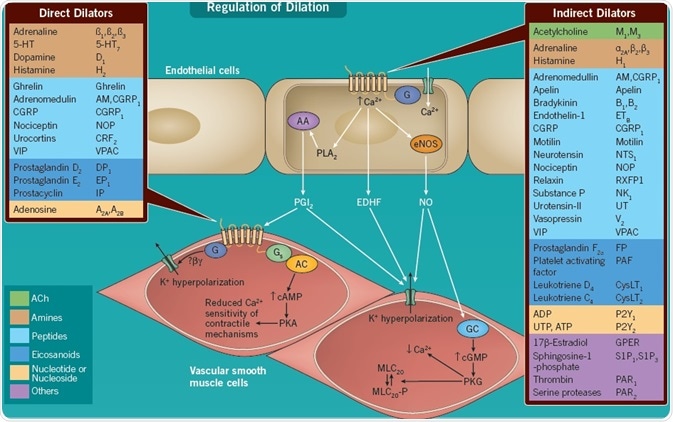
Indirectly Acting Vasodilators
As well as modulating coagulation and thrombus formation, the endothelium plays a contributory role in the modulation of vascular tone. It does this by responding to an array of physical factors, including shear stress, as well as chemical messengers, thus releasing endothelium-derived relaxing factors, such as prostacyclin (PGI2), endothelium-derived hyperpolarizing factor (EDHF) and NO[9].
In several instances, the chemical message undergoes transduction by GPCRs expressed on the surface of endothelial cells. These activate the release of relaxing factors, engendering dilation of the underlying smooth muscle. Receptor subtypes for the same transmitter may be expressed on both smooth muscle and endothelial cells.
In many instances, these endothelial cell receptors (including endothelin ETB receptors) can function as a feedback mechanism to resist vasoconstrictor responses mediated through a divergent subtype of the same receptor expressed on the underlying smooth muscle (e.g. endothelin ETA receptor)[10]. Contrastingly, although a single receptor, such as the apelin receptor, is expressed on vascular smooth muscle, it mediates its primary vasodilator effect through receptors associated with the release of endothelium-derived relaxing factors, located on endothelial cells[5].
Directly Acting Vasoconstrictors
The sympathetic nervous system, primarily via the release of noradrenaline from perivascular nerves, triggers smooth muscle α-adrenoceptors to constrict the vasculature tonically. Additional locally generated vasoconstrictors comprise 5-HT released from platelets, histamine from mast cells, and the peptide angiotensin II.
Moreover, the endothelium is the source of locally functioning endothelium-derived contracting factors, including urotensin-II, thromboxane A2, and ET-1, mediators that can resist the actions of endothelium-derived vasodilators to support the maintenance of basal tone[9]. Smooth muscle GPCRs also react to nucleotides and eicosanoids released locally from the perivascular nerves or endothelium, as well as to circulating constrictor hormones, such as adrenaline.
Directly Acting Vasodilators
Chemical messengers, which circulate in the plasma, as well as locally released mediators from endothelial cells or perivascular nerves, may also behave as directly acting vasodilators via interaction with GPCRs of both Class A and Class B expressed on smooth muscle.
In spite of their advantageous influence on the promotion of vasodilatation, receptors in Class B represent difficult targets for the development of orally active small molecule drugs possessing high-affinity and strong pharmacokinetic properties. A moderate number of CGRP receptor antagonists have undergone evaluation in early-phase clinical trials for migraine[8] but are yet to successfully reach the market.
References and Further Reading
- Alexander et al. (2015) Br. J. Pharmacol. 172 5744
- Katugampola et al. (2002) Clin. Sci. 103 171S
- Davenport et al. (2013) Pharmacol. Rev. 65 967
- Southern et al. (2013) J. Biomol. Screen. 18 599
- Yang et al. (2015) Trends Pharmacol. Sci. 36 560
- Maguire et al. (2011) PLoS One 6 e27601
- Kennedy et al. (2016) J. Am. Heart Assoc. 5 pii: e004421
- Bortolato et al. (2014) Br. J. Pharmacol. 171 3132
- Vanhoutte et al. (2015) Acta Physiol. (Oxf) doi:10.1111
- Davenport et al. (2016) Pharmacol. Rev. 68 357
About Tocris Bioscience
Tocris Bioscience is your trusted supplier of high-performance life science reagents, including receptor agonists & antagonists, enzyme inhibitors, ion channel modulators, fluorescent probes & dyes, and compound libraries. Our catalog consists of over 4,500 research tools, covering over 400 protein targets enabling you to investigate and modulate the activity of numerous signaling pathways and physiological processes.
We have been working with scientists for over 30 years to provide the life science community with research standards, as well as novel and innovative research tools. We understand the need for researchers to trust their research reagents, which is why we are committed to supplying our customers with the highest quality products available, so you can publish with confidence.
Tocris is part of the protein sciences division of Bio-Techne, which also includes the best in class brands R&D Systems, Novus Biologicals, ProteinSimple, and Advanced Cell Diagnostics. Bio-Techne has united these brands to provide researchers with a full portfolio of research reagents, assays, and protein platforms. For more information on Bio-Techne and its brands, please visit bio-techne.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.