Sponsored Content by SartoriusReviewed by Alex SmithJun 30 2022
The preparation of standards, also referred to as reference samples, of known concentrations is a frequently used procedure in most analytical laboratories. Internal or external standards with extremely low concentrations are employed in these laboratories for highly sensitive quantitative analytical methods to accurately establish the concentration of chemical components in samples.
With the application of highly sensitive quantitative analytical procedures, analytical methods are able to determine the concentration of chemical compounds in samples with great precision. External standards are separate samples applied for comparison to test samples, whereas internal standards are introduced to the samples to be analyzed.
Therefore, the accuracy of the concentration of such standards is extremely important to prevent subsequent errors when trying to establish unknown concentrations of compounds in samples. Two problems may merge when standards are prepared manually from soluble solids:
- The required solvent volume and the desired compound concentration are both used to determine the amount of a soluble compound to be weighed. The main problem is not in the calculation itself but the accuracy with which the amount of the solid is weighed relative to the calculated target quantity. On high-resolution laboratory balances, it is almost impossible to accurately weigh a compound to one μg or even less.
- If the amount weighed does not match the target amount calculated, the solvent quantity has to be modified to achieve the desired final concentration. Recalculation of the requisite liquid volume consumes valuable time and is a possible source of error.
Depending on the type of concentration specified, a number of parameters must be considered, such as the purity of the substance, desired concentration, amount actually weighed and potentially the molecular weight.
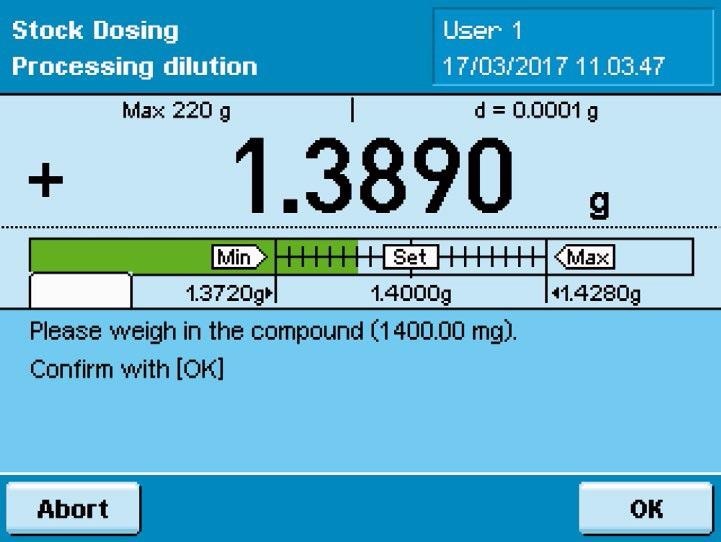
Image Credit: Sartorius
For users lacking experience, recalculation of the compound weight may consume a lot of time, whereas experienced users typically find this to be a boring task, so inadvertent errors can creep in easily.
The Cubis® MSA dosing system streamlines workflows in the preparation of standards. Once the user has entered the concentration and volume of a standard solution, the system automatically calculates the amount of compound required and displays the target weight as a bar graph with the minimum and maximum tolerances.
While the user will rarely end up weighing the exact amount in this case, the system software recalculates the volume of the solvent using the actual weight of the compound and transmits this value to the automatic dispenser.
Resultingly, the user will not need to recalculate the volume of solvent if any variations in the amount weighed from the desired target weight are noted when using the Cubis® MSA dosing system.
Instead, the workflow is able to progress without any interruptions, and the solvent can be dispensed. After the solvent has been introduced, the weight of the sample is once again established. In this way, the Cubis® balance validates how much solvent was actually added.
This gravimetric monitoring step is significantly more accurate than any volumetric measurement of a solvent. Volumetric measurement procedures are detrimental to the process in that they do not suitably account for effects on accuracy, such as evaporation of extremely volatile solvents, discrepancies in the density of solvents, and hold-up volume in tubing or pipettes, etc.
Conversely, gravimetric measurement methods offer absolute values and deliver the greatest levels of accuracy. At the end of the workflow, the dosing system software can calculate the precise concentration of the standard solution
When compared to other well-known, completely automatic dispensing systems on the market, the product detailed in this article offers a range of critical advantages. Completely automatic systems, which facilitate the dispensing of fine powder, granules and homogeneous or heterogeneous mixtures, necessitate total knowledge of the dispensing properties of these substances.
For instance, the flowability, shape, size, density, compactness, tendency to aggregate and electrostatic properties of the particles must either be known or evaluated visually. Selecting of the appropriate dispensing head is not always simple, given the numerous dosing parameters that may have only been qualitatively assessed.
Besides this, changing the accessories of the equipment, such as a dosing system to dispense a different compound each time, is a laborious, costly process as different dispensing heads need to be purchased.
It only makes sense to integrate systems such as this if an extremely high number of standards must be prepared. The semi-automatic system presented in this article offers greater flexibility and saves a significant amount of time to prepare a low or average number of standards.
Some of the reasons that call for an open-design system are the economic benefits and, by way of the system’s ease of use, reduced operating and maintenance costs.
Although the Cubis® MSA’s dosing system software offers the capacity to manage seemingly large numbers of data sets – up to 100 different compounds, solvents and standards – this number may still be too low for analytical laboratories that regularly prepare standards.
Additionally, the preparation of standards is just one part of the entire analytical procedure; therefore, centralized data management representative of the entire process chain is recommended.
To achieve these standards, the software of the Cubis® MSA dosing system is equipped with an interface for the ThermoFisher Chromeleon™* 7.2 SR4 chromatography data system that has been developed to manage chromatographic systems and carry out data analysis.
In the Chromeleon component table, the user defines the desired concentration of standard samples:
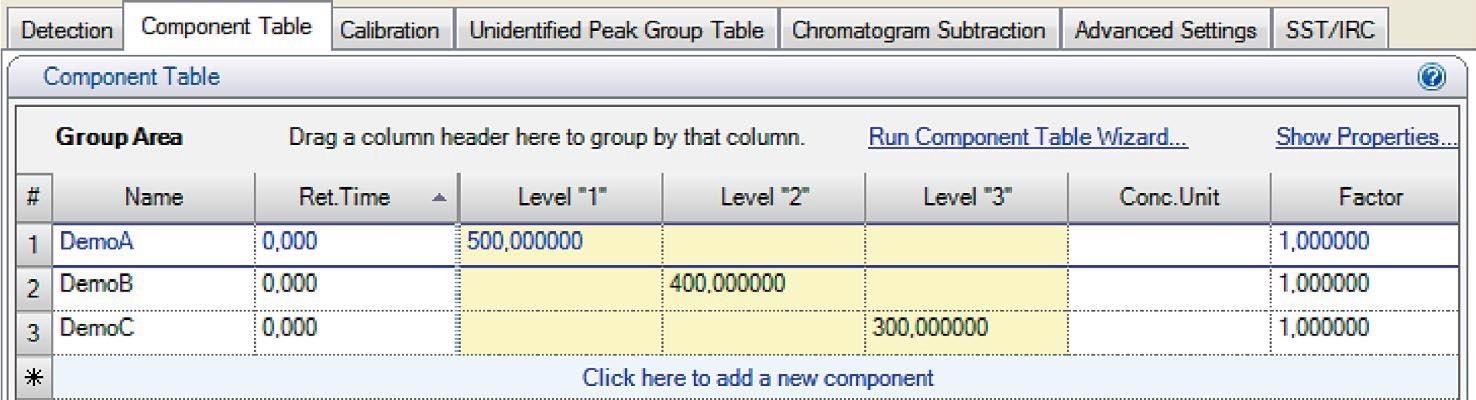
Image Credit: Sartorius
When activating the command “Send weighing input to scale,” data, including the desired concentration and the name of the standard, is sent to the Cubis® MSA dosing system.
This desired concentration is then used to calculate the amounts to be weighed by the integrated software application, which subsequently shows these values to the user for validation and then starts the weighing and dispensing procedure.
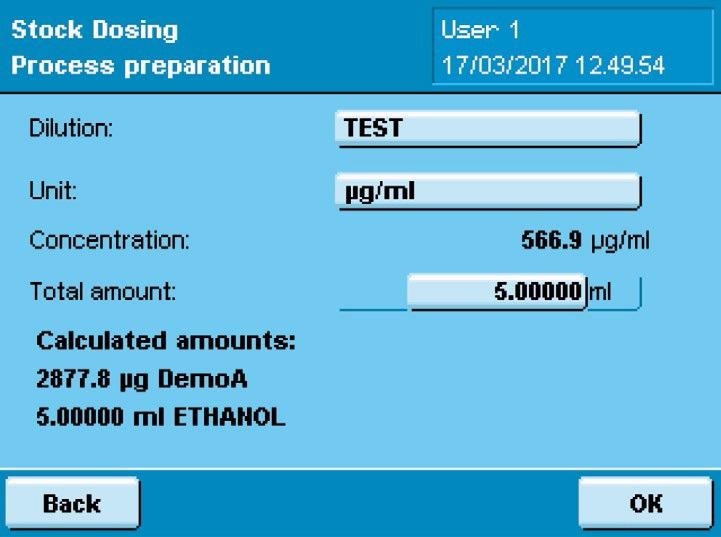
Image Credit: Sartorius
This process repeats until all the standard samples that the Chromeleon has transmitted have been processed. Using the function “Retrieve weighing result from scale,” previously saved data is retrieved from the balance and transmitted back to Chromeleon.
The confirmed concentrations of the standards and the unit are saved by Chromeleon and later used for evaluation. This direct communication interface guarantees the highest level of data integrity as manual inputs are removed from the process to exchange data.
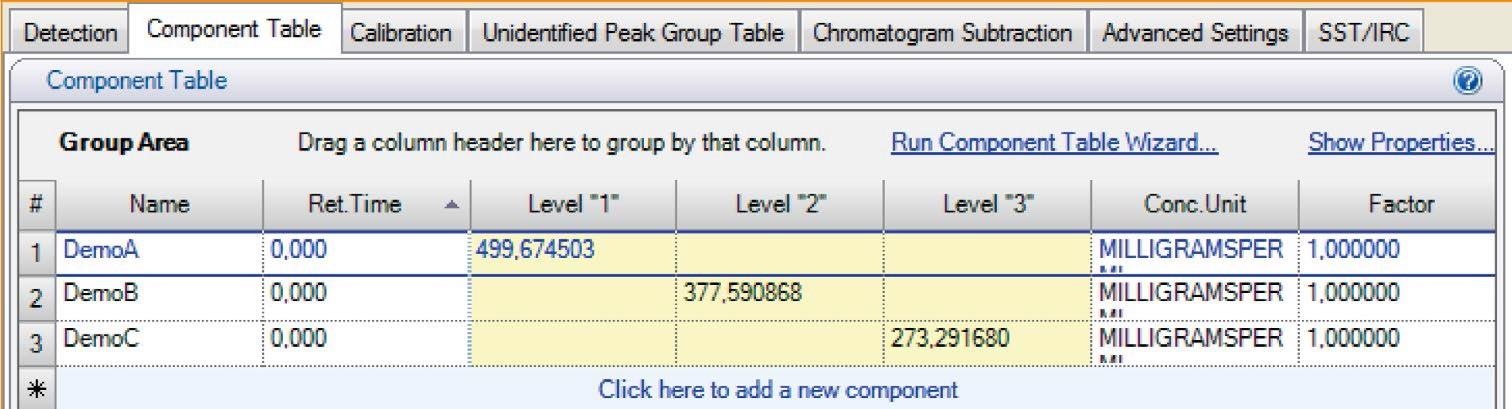
Image Credit: Sartorius
Chromeleon* 7.2 SR4 facilitates complete automated data management of any desired number of standards.
It is recommended to connect the Cubis® MSA dosing system to Chromeleon when expanding any analytical laboratories’ capabilities for automation of workflows. Ultimately, automation means that a significant amount of time is saved compared with manual data exchange while enhancing the reliability of the process.
* Dionex™ Chromeleon™ 7.2 Chromatography Data System (CDS) software is a trade mark of company Thermo Scientific™
About Sartorius

Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative technologies enable medical progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making lab life easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.