Antibody-drug conjugates and monoclonal antibodies (mAb) are extensively used biological therapeutics. A highly crucial property is the rate and extent of internalization, governing efficacy, safety, and PD profile. Therefore, it is vital to quantify and compare Ab internalization in the biopharmaceutical process.
This article describes an innovative, automated, and feasible cell-based Ab internalization assay that is medium-throughput, ready to use, and geared toward industrial biologies discovery. Incucyte® Live-Cell Analysis is used to perform internalization measurements on 96-well microplates over time.
mAbs were labeled and tested by coupling an antibody-binding fragment with a pH-sensitive dye (FabFluor-pH), through a single-step, no-wash protocol. As predicted, there was an increase in fluorescence signal as the mAb complex was internalized into the acidic lysosome.
This methodology has been verified for use in the temporal and pharmacological characterization of mAb. Presented here is a proof of concept of a screening cascade to compare Ab characteristics and signal specificity across epitopes and cell lines.
Concentration-dependent internalization of clinical antibodies
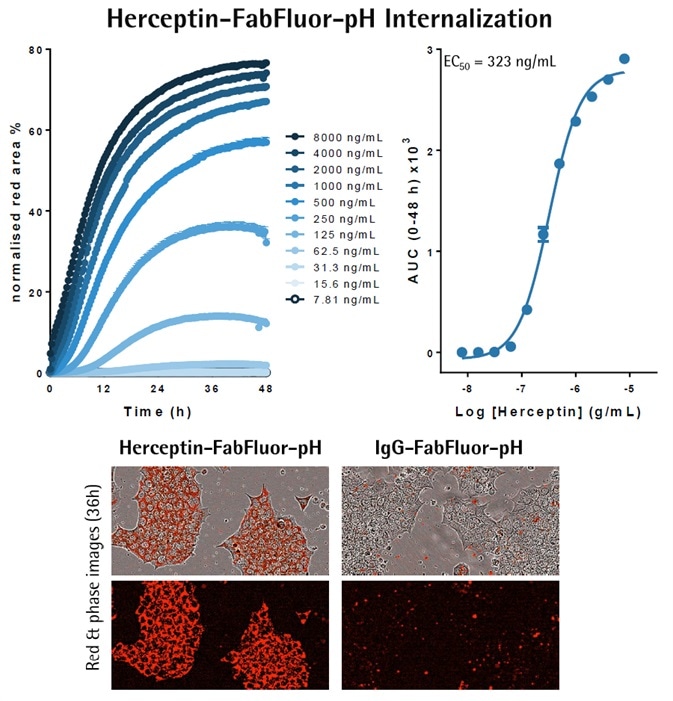
Image Credit: Incucyte®
- Incucyte® FabFluor-pH labeled-Flerceptin or IgG, isotype control, was used to treat BT-474 Her2-positive breast cancer cells (10K/well).
- Red fluorescence and phase images were captured (10x objective) every 30 minutes over a period of 48 hours.
- Measurement of the red fluorescence area, when normalized for phase area (confluence), shows a fast, concentration-dependent increase in signal over time, with labeled Herceptin but no signal with IgG.
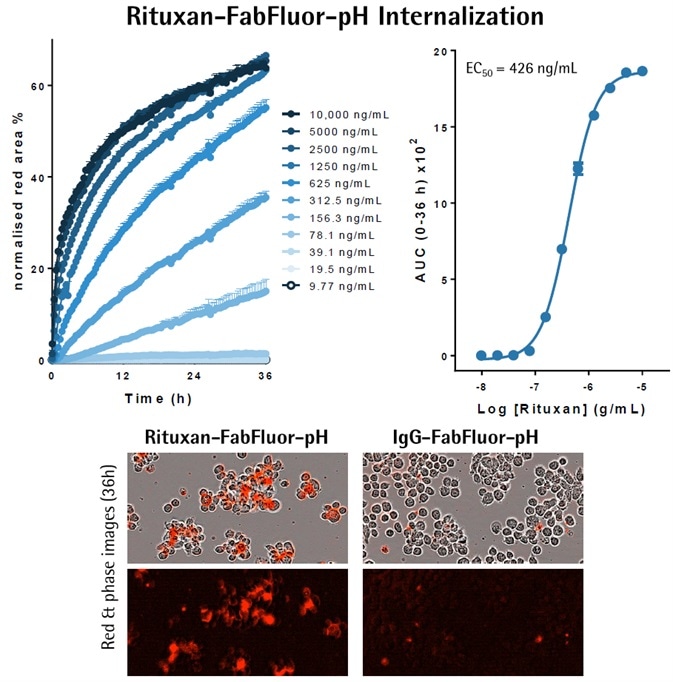
Image Credit: Incucyte®
- Either Incucyte® FabFluor-pH labeled-Rituxan or IgG, isotype control, was used to treat Raji cells (30K/well).
- Red fluorescence and phase images were captured (20x objective) every 30 minutes over a period of 36 hours.
- Measurement of the red fluorescence, when normalized for phase area (confluence), shows a fast, concentration-dependent increase in signal over time, with labeled Rituxan but no signal with IgG.
Drug discovery screening application
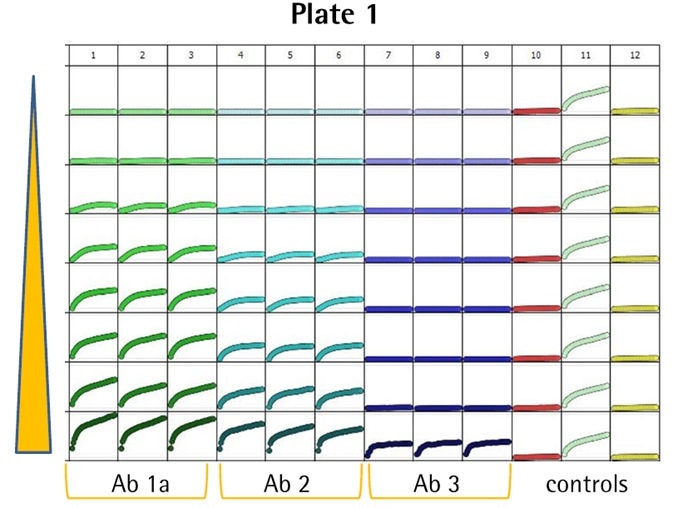
Image Credit: Incucyte®
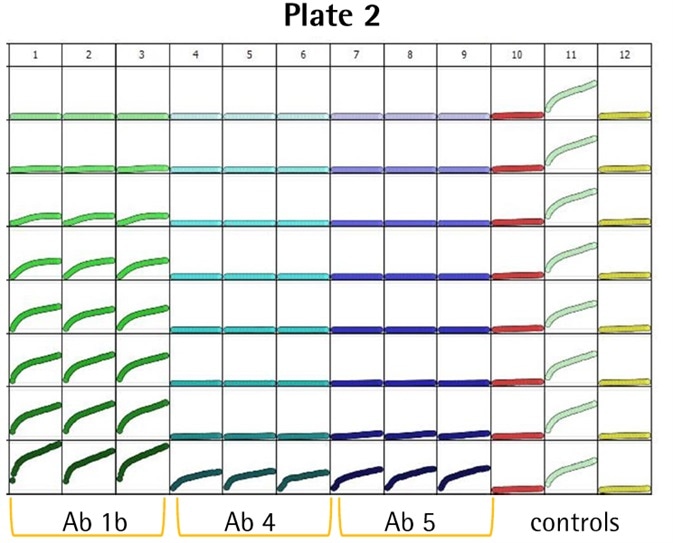
Image Credit: Incucyte®
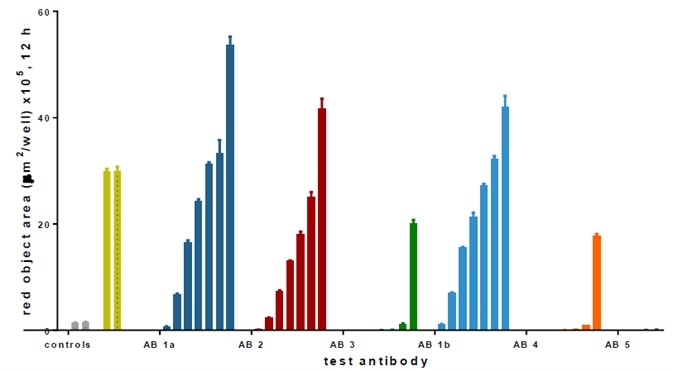
Image Credit: Incucyte®
- Several anti-CD71 Abs were labeled and internalization characteristics were compared (triplicate wells, CRC) in HT1080 fibrosarcoma cells (8K/well).
- Positive control wells (α-CD71) exhibited an evident increase in fluorescence, while little or no signal was noticed in the negative controls (IgG isotype) — Z′ values of 0.75 and 0.87.
- Among the six Abs, three (i.e., Ab 1a, 2, and 1b) generated a huge signal and were detected at low concentrations (<0.05 μg/mL).
- Ab 1a and 1b were the Ab clone obtained from different suppliers, and as predicted, they generated similar responses.
- Abs 3, 4, and 5 were internalized only at higher test concentrations.
Receptor expression specificity
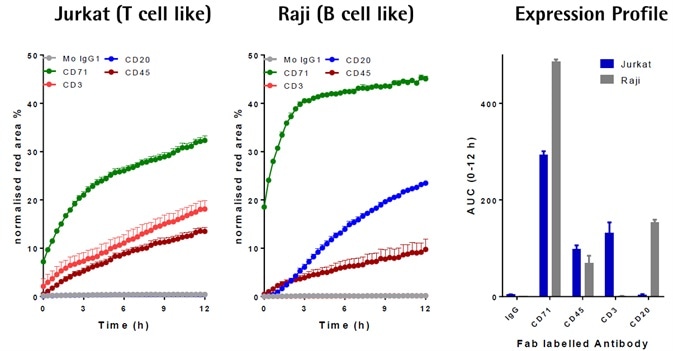
Image Credit: Incucyte®
- Different FabFluor-pH-Abs were added to Raji or Jurkat cells (4 μg/mL).
- CD71 and CD45 (general lymphocyte markers) were internalized by both cell lines CD3 (T cell marker), and not CD20, was internalized by only Jurkat, which was in contrast to the Raji cells (B cells).
- In these cell lines, data was in correlation with known CD surface marker expression, therefore offering high confidence in the generic utility and signal specificity of the methodology.
Continuous live-cell analysis: Methodology

Image Credit: Incucyte®
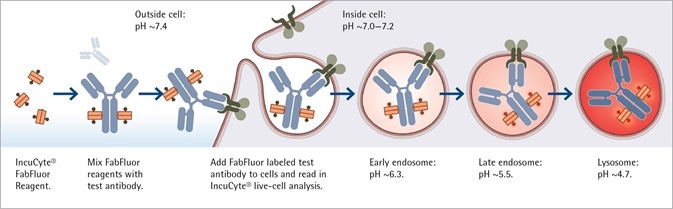
Image Credit: Incucyte®
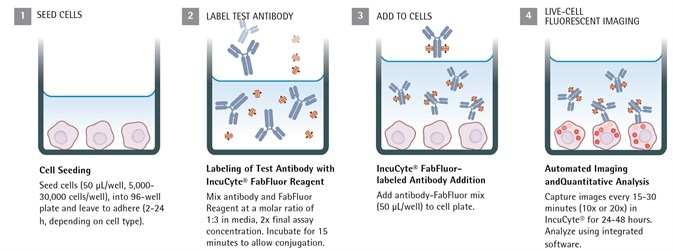
Image Credit: Incucyte®
Antibody internalization with Incucyte® FabFluor-pH reagent
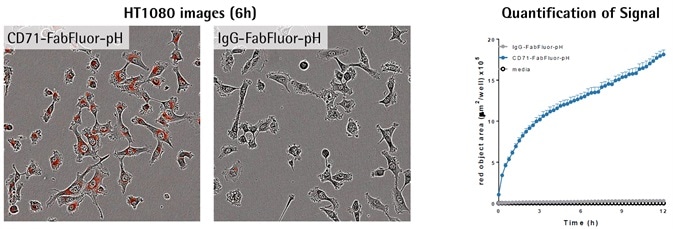
Image Credit: Incucyte®
- Images exhibit red fluorescence in the cytoplasm of cells treated using α-CD71, but not IgG1, which shows a particular internalization signal.
- Measurement of the red signal (fluorescence area) exhibits a fast increase in red object area over time with labeled α-CD71 only.
Signal amplitude is cell number-dependent
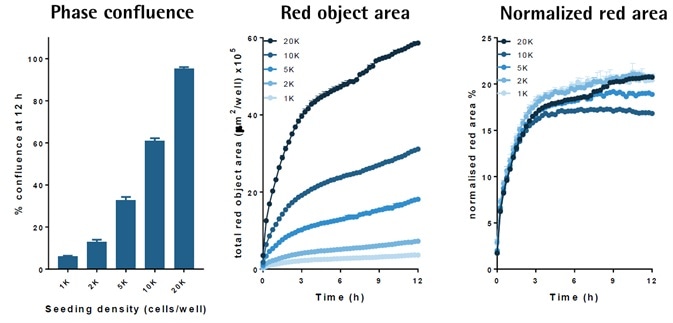
Image Credit: Incucyte®
- FabFluor-pFI-α-CD71 (4 μg/mL) was used for treating a higher density of FIT1080 cells.
- Time-course data indicates an increase in the size of the internalization signal when the cell density increased.
- Normalization of the signal area for cell density (phase area) led to an overlay of the responses, indicating a similar internalization rate.
- The data reveals that the signal size depends on cell number but not the internalization rate.
Lysosomal localization of internalization signal
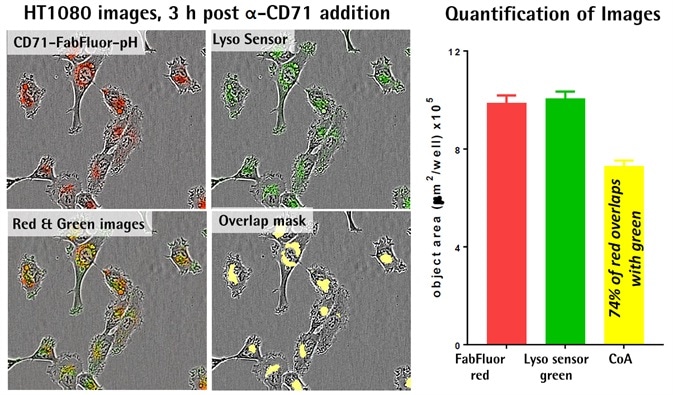
Image Credit: Incucyte®
- FabFluor-pFI-α-CD71 (4 μg/mL) internalization was established prior to the addition of Lyso Sensor® green (Thermo, 0.25 μM).
- The captured images display the cellular localization of the internalization signal (red), masking of the co-localized signal, overlaid images, and the lysosome marker (green).
- Quantification displays a similar area of both green and red signal within the cells.
- The coincident object analysis (COA) feature was used to predict that 74% of the red area was co-localized with the green.
- The data indicates the internalization of the antibody into lysosomes.
Acknowledgments
Produced from materials originally authored by N. Bevan, T. Dale and D. Trezise (sponsor Daniel Martinez Molina) from Essen BioScience.
About Incucyte®
The Incucyte® Real-Time Quantitative Live Cell Analysis System, designed by Essen BioScience, Inc (now a Sartorius company) is the first system to continuously quantify cell behavior over time (from hours to weeks) while cells remain undisturbed inside a standard incubator. The Incucyte® System automatically collects and analyzes images continuously around the clock, providing insight into active biological processes that is difficult to achieve with endpoint assays. The Incucyte® suite of assays utilize proprietary reagents that do not perturb cell health with validated 96/384 well format protocols that save time and money.
Incucyte® Live-Cell Analysis has revolutionized numerous studies, with applications such as stem cell monitoring and reprogramming, label-free analysis, ATP metabolism, multiplexing, neurite outgrowth and dynamics, migration/invasion, 3D-spheroids, angiogenesis, NETosis, reporter gene expression, immunocytochemistry, immune cell killing, apoptosis, cytotoxicity, kinetic data generation, antibody internalization. All this without the removal of cells from an incubator.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.