Sponsored Content by SartoriusReviewed by Sarah KellyOct 27 2025
Apoptosis, or programmed cell death, is a tightly regulated biological process necessary for normal tissue maintenance and development. However, aberrations in apoptotic signaling networks are implicated in a range of human diseases, including autoimmune disease, neurodegeneration, and cancer.1
Apoptotic pathways can be initiated by external or internal cues. Extrinsic pathways are initiated by the activation of pro-apoptotic receptors on the cell's surface, while intrinsic pathways arise from a wide range of stimuli, such as hypoxia, the absence of growth factors, defective cell cycle control, DNA damage, or other types of cellular stress that lead to the release of cytochrome C from mitochondria.
In the majority of cases, the induction of apoptosis leads to the activation of a family of proteins known as caspases (cysteinyl aspartate proteinases). For example, caspase-3 or caspase-7 activation often results in the irreversible commitment of the cell to apoptotic death and is therefore widely regarded as a dependable marker for apoptosis.2
Another classical marker of apoptosis is the regulated loss of plasma membrane phosphatidylserine (PS) symmetry. Dying cells catalyze the translocation of the normally inward-facing PS to the cellular surface, enabling early phagocytic recognition of the dying cell by the surrounding phagocytes.3
A number of plate-reader, enzymatic, and flow-cytometric assays have been developed to measure PS externalization or caspase-3/7 activation. Most caspase-3/7 assays feature colorimetric, luciferase, or fluorometric reagent substrates incorporating a DEVD (Asp-Glu-Val-Asp) peptide motif recognizable by the enzyme.4
Similarly, Annexin V is a recombinant protein employed in apoptosis detection due to its high selectivity and high affinity for PS residues. Apoptosis assays leveraging Annexin V conjugated to a fluoroprobe have been optimized for the detection of PS externalization. These assays are typically measured using flow cytometry.5
Despite their utility, these common apoptosis assays suffer from a series of disadvantages, including:
- Their capacity to yield only a single user-defined endpoint measurement.
- The need to employ multiple wash steps or cell lifting that can result in a loss in PS asymmetry or the loss of dying cells.
- A lack of amenability to long-term measurements due to an increasing background signal over time.
While live-cell imaging and analysis methods have been successfully developed to offer a more dynamic view of apoptosis, optimized technology and reagents are necessary to ensure the accurate and flexible assessment of cell death.6
This article explores the use and value of Incucyte® Apoptosis Assays, incorporating no-wash, mix-and-read reagents and integrated image-based analysis tools that have been optimized for the kinetic quantification of apoptotic activity.
Assay principle
The Incucyte® Apoptosis Assays leverage Incucyte® Caspase-3/7 and/or Annexin V Dyes alongside the Incucyte® Live-Cell Analysis System to facilitate the automated, real-time measurement of apoptosis.
Incucyte® Caspase-3/7 Dyes are non-fluorescent (DEVD), inert substrates able to freely cross the cell membrane. Once there, these substrates can be cleaved by activated caspase-3/7 to release a red, green, or orange (for metabolism) DNA-binding fluorescent label.7 Figure 1A shows the identification of apoptotic cells via the appearance of fluorescently labeled nuclei.
Incucyte® Annexin V Dyes are labeled with extremely bright and photostable cyanine fluorescent (CF) dyes designed to emit a red, green, orange, or near-infrared (NIR) signal on binding to exposed PS in apoptotic cells (Figure 1B). The use of intuitive integrated analysis software enabled the quantification of these fluorescent objects with minimal background signal.
These apoptotic signals were correlated with Incucyte® high-definition phase-contrast images to gain further biological insight and morphological validation of apoptotic features, including membrane blebbing, nuclear condensation, cell shrinkage, and DNA fragmentation.
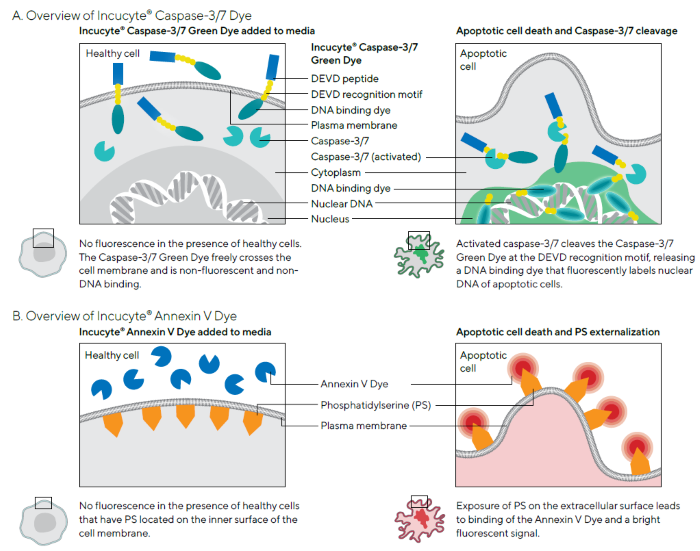
Figure 1. Schematics Demonstrating Principles of Incucyte® Apoptosis Assays. Image Credit: Sartorius
Materials and method
The Incucyte® Apoptosis Assay can be performed using a simple mix-and-read protocol in a high-throughput 96/384-well format (Figure 2). This highly flexible assay enables noninvasive real-time analysis of treatment effects and is suitable for use with a range of adherent and non-adherent cells.
Additionally, apoptosis measurements can be multiplexed with measurements of cytotoxicity or proliferation by combining these with Incucyte® Nuclight Reagents for nuclear labeling, the Incucyte® Confluence metric, or Incucyte® Cytotox Dyes.
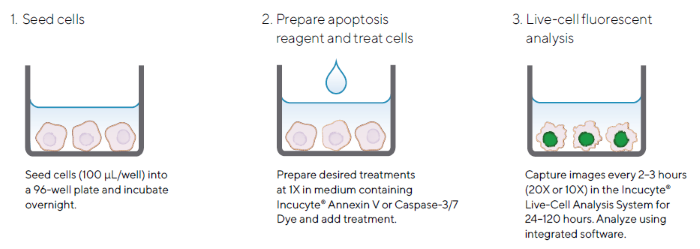
Figure 2. Quick Guide of Incucyte® Apoptosis Assay Protocol for Adherent Cells. Image Credit: Sartorius
Note. The simple protocol utilizes Incucyte® Caspase-3/7 or Annexin V Dyes and the Incucyte® Live-Cell Analysis System for image-based fluorescent measurements of apoptosis.
Validation data
Quantification of apoptosis
When used in conjunction with apoptosis readouts, the Incucyte® Live-Cell Analysis System enables the real-time visualization and quantification of caspase-3/7 activity or PS exposure in response to pharmacological treatment.
For example, HT-1080 fibrosarcoma cells were treated with the anti-cancer drug Cisplatin (CIS; 12.5 µM) in the presence of Incucyte® Annexin V Red Dye, exhibiting a kinetic increase in fluorescence (Figure 3).
Apoptosis quantification was possible over the entire assay time-course due to integrated image-based analysis tools, supported by the automatic segmentation of fluorescence (mask shown in blue). The acquired images also enabled the correlation of apoptotic readouts with the morphological changes linked to cell death.
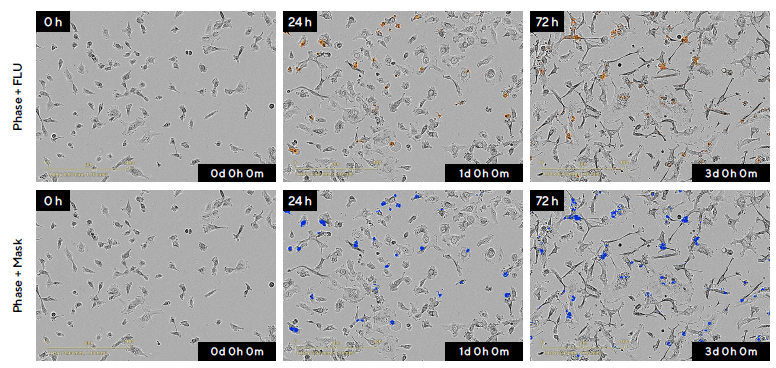
Figure 3. Visualization and Quantification of HT-1080 Fibrosarcoma Cells in Response to the Anti-Cancer Drug Cisplatin (CIS) in Real-Time. Image Credit: Sartorius
Note. HT-1080 cells were seeded at a density of 2000 cells per well and treated after 18 hours with 12.5 μM CIS in the presence of Incucyte® Annexin V Red Dye. Using the Incucyte® Live-Cell Analysis System, high-definition images (20X magnification) were acquired every two hours for the duration of the experiment.
Representative phase-contrast and blended fluorescence images (top row) and the segmentation mask (blue) generated using integrated analysis software (bottom row) are shown over time (0 to 72 hours). A progressive increase of PS binding, indicated by Incucyte® Annexin V Red Dye, was observed following drug treatment.
The Incucyte® Annexin V Red fluorescent signal can be aligned with morphological changes associated with cell death, including noticeable cell shrinkage and membrane blebbing.
Pharmacological and kinetic analyses of apoptosis
The Incucyte® Apoptosis Assay facilitates high-throughput investigation of apoptosis in response to compound treatments. A pharmacological study was performed on A549 cancer cells to illustrate the amenability of this approach to drug toxicity testing.
In this example, cells were treated with two-fold serial dilutions of four different compounds in the presence of Incucyte® Annexin V NIR Dye and fluorescent objects. These cells were then analyzed automatically using integrated software.
It was observed that there was a kinetic increase in fluorescence for all compounds. This was shown in the microplate view with variable profiles noted for the different compounds (Figure 4A).
Camptothecin (CMP), cisplatin (CIS), and staurosporine (SSP) were shown to exhibit concentration-dependent effects, while nocodazole (NCD) exhibited low levels of apoptosis across all concentrations tested.
Figure 4B shows the kinetic apoptotic response to CMP, a DNA synthesis inhibitor that is widely used as a research tool to induce apoptosis.8 Transformation of the kinetic data at 72 hours into a concentration-response curve highlights a concentration-dependent effect on A549 cells. No increase in fluorescence was observed in control conditions.
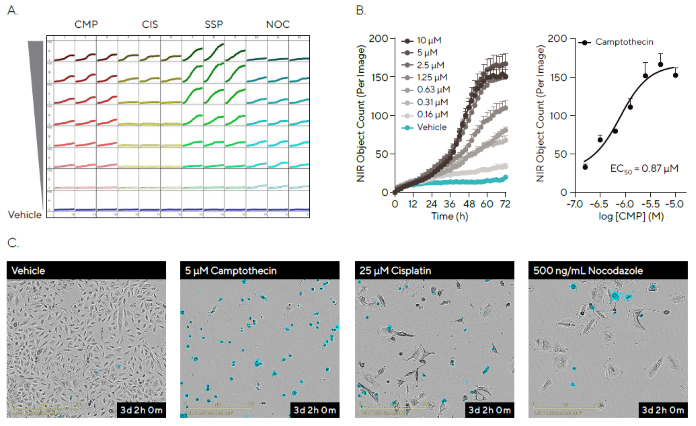
Figure 4. Pharmacological Analysis of Apoptosis in a High-throughput Manner. Image Credit: Sartorius
Note. A549 cells were seeded at a density of 2000 cells per well and treated after 18 hours with a range of concentrations of four compounds in the presence of Incucyte® Annexin V NIR Dye.
(A) The Incucyte® Apoptosis Assay allows automated imaging and quantitative analysis of every well of a 96/384-well plate, generating a microplate readout of apoptosis over time. The microplate view shows kinetic change in fluorescence following compound treatment, with distinct response patterns observed as measured by NIR Object Count.
(B) Time course data shows the concentration-dependent kinetic effect of Camptothecin (CMP; 10–0.16 μM). Further analysis reveals a concentration-response curve at 72 hours for CMP.
(C) Representative phase-contrast and NIR fluorescent images (pseudo-colored blue, 20X; 72 hours) show that apoptotic fluorescence signals correspond with morphological changes. Data represented as Mean ± SEM, n = 3.
Representative phase-contrast and blended fluorescent images highlighted the morphological changes linked to apoptosis, enabling the qualitative comparison of different drug treatments to vehicle conditions (Figure 4C).
These results show the benefits of the Incucyte® Apoptosis Assay in the quantification and visualization of drug-induced apoptosis while demonstrating the amenability of live-cell analysis to high-throughput pharmacological investigation.
Multiplexed measurements of proliferation and apoptosis
It is also possible to combine Incucyte® Apoptosis Assays with Incucyte® Nuclight Reagents for nuclear labeling to enable the multiplexed measurements of proliferation and apoptosis via the Incucyte® Live-Cell Analysis System.
To demonstrate this approach, HT-1080 fibrosarcoma cells labeled with Incucyte® Nuclight NIR were treated with a three-fold serial dilution of CMP in the presence of Incucyte® Caspase-3/7 Dye (Figure 5).
Representative phase and blended fluorescence images revealed a reduction in NIR nuclear fluorescence (pseudo-colored blue) and an increase in green fluorescently stained DNA. This indicates Caspase-3/7 activation following CMP treatment.
It was also possible to use integrated software to automatically mask fluorescence and quantify cell proliferation (NIR) and cell death (green). Quantification revealed that CMP had a kinetic concentration-dependent apoptotic and anti-proliferative effect on HT-1080 cells.
This data shows the ability of two-color kinetic assays to offer a multi-parametric approach for analyzing the apoptotic and anti-proliferative effects of pharmacological treatments. It also highlights this approach’s potential for the investigation of novel compounds in drug discovery.
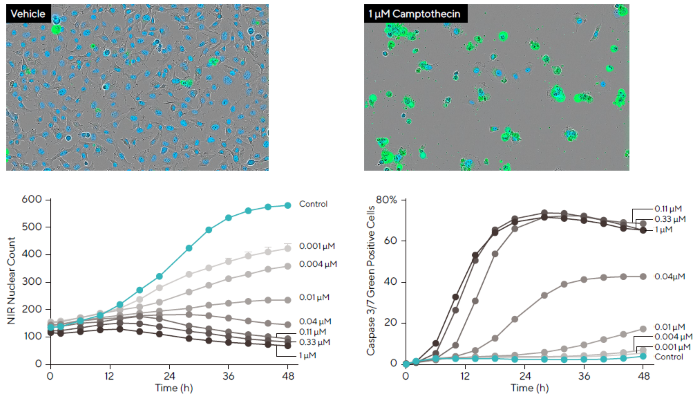
Figure 5. Investigation of Compound Effects Using Multiplexed Measurements of Apoptosis and Proliferation. Image Credit: Sartorius
Note. HT-1080 fibrosarcoma cells stably expressing Incucyte® Nuclight NIR Lentivirus (pseudo-colored blue) were exposed to a dilution series of CMP (1–0.001 μM) in the presence of Incucyte® Caspase-3/7 Green reagent.
Over a 48-hour period, a concentration-dependent decrease in nuclear count was detected, alongside a corresponding increase in cell death. Representative phase-contrast and fluorescent images (10X magnification) captured at 48 hours validate kinetic data of both cell viability and apoptosis.
Data presented as Mean ± SEM, n = 12.
Assessment of cell health in subpopulations of cells
The Incucyte® Cell-by-Cell Analysis Software Module can facilitate the segmentation of individual cells in the phase image and the extraction of metrics per cell relating to fluorescence within the segmented boundary.
Integrated software analysis enables the classification of cell populations based on fluorescence characteristics. When leveraged in combination with multiplexed assays, this capability allows the rapid quantification of the cell health of sub-populations.
HT-1080 fibrosarcoma cells stably expressing Incucyte® Nuclight Red (nuclear viability marker) were treated with either cytostatic cyclohexamide (CHX) or cytotoxic camptothecin (CMP) in the presence of Incucyte® Caspase-3/7 Green Dye in order to exemplify this (Figure 6).
Images were acquired every two hours, with Cell-by-Cell Analysis used to classify cell subsets on the basis of red and green fluorescence intensity. Representative phase and fluorescence images and classification plots for each compound show cells classified as red, red and green, or green.
These subpopulations were temporally tracked, revealing that CMP induced a decrease in the red population, indicating a loss of viable cells. This was accompanied by an increase in the red and green population, indicating early apoptosis, and an increase in the green population, indicating late apoptosis after more than 24 hours.
Time courses acquired of the early apoptotic population (red and green) revealed a concentration-dependent apoptotic response to CMP in HT-1080 cells. These findings were consistent with a cytotoxic mechanism of action. CHX treatment did not induce apoptosis at any of the tested concentrations, but a constant red population (viable) was maintained over time. This was consistent with the cytostatic mechanism of action.
Data showed that the combination of Incucyte® Cell-by-Cell Analysis and multiplexed assays can be used to acquire dynamic insight into drug-induced treatment effects on cell health, and that this approach is suitable for the identification of therapeutic mechanisms on a specific cell type.
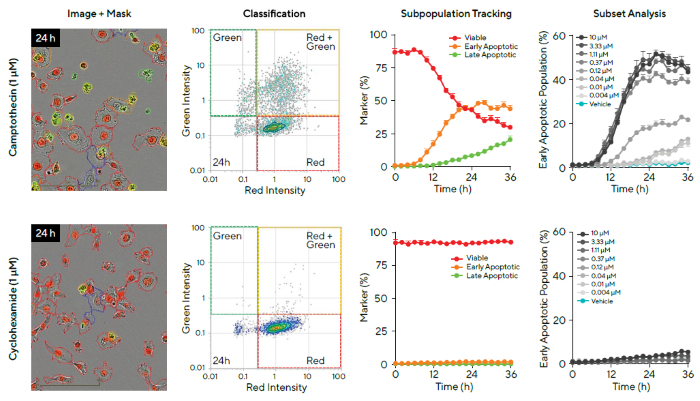
Figure 6. Investigation of Cell Health of Subpopulations of Cells Following Different Compound Treatments. Image Credit: Sartorius
Note. HT-1080 fibrosarcoma cells stably expressing Incucyte® Nuclight Red (nuclear viability marker) were treated with Camptothecin (CMP) or Cyclohexamide (CHX) in the presence of Incucyte® Caspase-3/7 Green.
Cell images show response at 24 hours with associated classification masking (first column). Using Incucyte® Cell-by-Cell Analysis Software, cell subsets were classified based on red and green fluorescence intensity (second column).
Following exposure to CMP, there was a decrease in the red population (viable cells), an increase in red and green population (early apoptosis), and green-only cells (late apoptosis) (third column, top). In contrast, after CHX treatment, there was a lack of apoptosis (third column, bottom).
Concentration-response time courses of the early apoptotic population are shown (percentage of total cells exhibiting red and green fluorescence, fourth column).
Data presented as Mean ± SEM, n = 3.
Summary and outlook
Apoptosis is a complicated, dynamic process that has been implicated in a number of pathologies. A deeper understanding of apoptosis mechanisms is essential across multiple therapeutic areas.
The combination of Incucyte® Caspase-3/7 or Annexin V Dyes and real-time image analysis via the Incucyte® Live-Cell Analysis System can be used to generate a quantitative picture of this changing landscape.
The Incucyte® Apoptosis Assays offer a full kinetic apoptotic signaling readout via caspase-3/7 activity or PS exposure in physiologically relevant conditions. These assays offer beneficial insight into the dynamics and timing of the apoptotic signaling pathway, eliminating the need to determine a single assay endpoint. These endpoints can vary greatly between cell types and compound treatment conditions.
This approach also allows data to be validated using acquired images, as well as assessment of the morphological changes linked to cell death. It is possible to gain further insight via multiplexing apoptosis readouts with measurements of proliferation. Using these measurements in combination with Incucyte® Cell-by-Cell Analysis allows the tracking of individual subpopulations of cells’ cell health.
These assays offer a novel and flexible method that is amenable to a range of experimental set-ups (Table 1), facilitating apoptotic pathway analysis for both basic cell biology research and drug discovery.
Table 1. Incucyte® Dyes for Live-Cell Imaging and Analysis of Cell Health. Source: Sartorius
|
Incucyte®
Cytotox Dye |
Incucyte®
Annexin V Dye |
Incucyte®
Caspase-3/7 Dye |
| Cell viability |
✓ |
|
|
| Apoptosis |
|
✓ |
✓ |
| 2D Monolayer + multiplex |
✓ |
✓ |
✓ |
| 3D Single spheroid |
✓ |
✓ |
|
| 3D Multi-spheroid + multiplex |
✓ |
✓ |
|
| Fluorescence channels |
Red, green,
near-IR |
Red, green,
orange, near-IR |
Red, green,
orange* |
*Incucyte® Metabolism
Note. Table summarizes Incucyte® Dyes validated for investigation of cell health using the Incucyte® Live-Cell Analysis System across a range of assay formats.
References and further reading
- Cotter, T.G. (2009). Apoptosis and cancer: the genesis of a research field. Nature Reviews Cancer, (online) 9(7), pp.501–507. https://doi.org/10.1038/nrc2663.
- Shi, Y. (2002). Mechanisms of Caspase Activation and Inhibition during Apoptosis. Molecular Cell, 9(3), pp.459–470. https://doi.org/10.1016/s1097-2765(02)00482-3.
- Tyurina, Y.Y., et al. (2000). Phospholipid signaling in apoptosis: peroxidation and externalization of phosphatidylserine. Toxicology, 148(2-3), pp.93–101. https://doi.org/10.1016/s0300-483x(00)00199-2.
- Thornberry, N.A., et al. (1997). A Combinatorial Approach Defines Specificities of Members of the Caspase Family and Granzyme B. Journal of Biological Chemistry, 272(29), pp.17907–17911. https://doi.org/10.1074/jbc.272.29.17907.
- Crowley, L.C., et al. (2016). Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harbor Protocols, 2016(11), p.pdb.prot087288. https://doi.org/10.1101/pdb.prot087288.
- Daya, S., et al. (2010). Integrating an automated in vitro combination screening platform with live-cell and endpoint phenotypic assays to support the testing of drug combinations. Available at: https://biotium.com/wp-content/uploads/2015/06/Essen_AstraZeneca_2010SBS_Poster_Apoptosis.pdf.
- Cen, H., et al. (2008). DEVD-NucView488: a novel class of enzyme substrates for real-time detection of caspase-3 activity in live cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology, (online) 22(7), pp.2243–2252. https://doi.org/10.1096/fj.07-099234.
- Ulukan, H. and Swaan, P.W. (2002). Camptothecins. Drugs, 62(14), pp.2039–2057. https://doi.org/10.2165/00003495-200262140-00004.
Acknowledgments
Produced from materials originally authored by Jasmine Trigg, John Rauch, Libby Oupicka, Clare Szybut, Nicola Bevan, and Kalpana Barnes from Sartorius.
About Sartorius
Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative technologies enable medical progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making lab life easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.