Cytotoxicity
The detrimental effects caused by substances or environmental changes on cell health are described as cytotoxicity. When cells are exposed to a cytotoxic stimulus, their metabolic activity may be compromised and cell growth or division may be inhibited, eventually leading to cell death.
Apoptotic cell death involves a more controlled and programmed mechanism, whereas necrotic cell death is a catastrophic cell lysis. A specialized process involving digestion of cells by themselves from within is called autophagy. Cell death is inevitable when there is an irreversible loss of membrane integrity for a cell.
Cytotoxicity assays
In most cytotoxicity assays, cell membrane integrity is measured either by markers released from dying cells (for instance, cellular proteases) or with vital dyes that are removed from healthy cells, for instance, propidium iodide or trypan blue.
Cell viability and health is measured using metabolic activity measurements, for instance ATP, LDH, or MTT assays. However, only a few methods are available that can directly count the number of dying cells over time.
IncuCyte® cytotoxicity assay
Real-time, automated cytotoxicity assays can be achieved within a tissue culture incubator using the IncuCyte® s3 live-cell imaging and analysis system. Using the IncuCyte® Cytotox reagents, cell death is measured in real-time based on cell membrane integrity by the IncuCyte™ Cytotoxicity Assay, all within a tissue culture incubator.
- IncuCyte® Cytotox Red Reagent - labels dying cells red
- IncuCyte® Cytotox Green Reagent - labels dying cells green
The IncuCyte® Cytotox reagents are non-fluorescent, inert, and do not enter healthy cells when introduced into the tissue culture growth medium. However, the Cytotox probe enters death cells due to the loss of membrane integrity, fluorescently labeling the nuclei.
Based on the appearance of red (or green) labeled nuclei, the probe allows identification and quantification of dying cells over time. Cell death can be further validated based on morphology using high definition phase contrast movies and images (for example, loss of motility and loss of cytoskeleton structure).
Real-Time Detection of Cytotoxicity Using the IncuCyte® Live-Cell Imaging System
Real-time detection of cytotoxicity using the IncuCyte® Live-Cell Imaging System.
Real-time analysis of neuronal cell death
Real-time analysis of neuronal cell death.
IncuCyte® cytotoxicity assay concept
- The addition of mix-and-read IncuCyte® Cytotox reagents to cultures allows cytotoxicity to be detected in real-time
- Dying cells can be automatically quantified using intuitive IncuCyte® image analysis tools
- Multiplex cytotoxicity readouts with measurements of apoptosis or proliferation when used in combination with the IncuCyte® Caspase 3/7 Reagent or the IncuCyte® NucLight nuclear labeling reagents
Key advantages of the IncuCyte® cytotoxicity assay
The following are the key advantages of the IncuCyte® cytotoxicity assay:
- Visualize and quantify cytotoxicity using time-lapse imaging
Cell death can be observed over time and cytotoxicity can be measured with intuitive IncuCyte® image analysis tools.
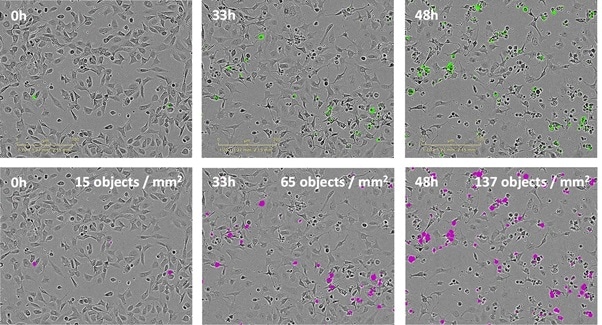
Figure 1. Visualize and validate cytotoxicity with images and movies. Time lapse images of SK-OV-3 ovarian cancer cell death in response to the anti-cancer drug camptothecin. Correlate fluorescent signal from the IncuCyte® Cytotox Reagents with morphological changes associated with cell death. Quantify cytotoxicity using IncuCyte® image analysis tools. User-friendly software enables direct image-based detection of dying cells (pink mask).
- Automatic analysis of the time course of cell death within an incubator
The mechanism and time course of treatment effects can be determined without removal of cells from the stable incubator environment. The IncuCyte® cytotoxicity assay is ideally suited for long-term studies with duration from 0 day to more than 10 days.
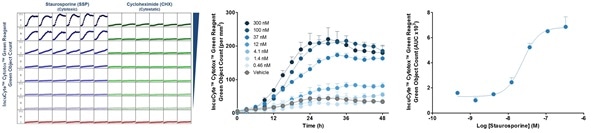
Figure 2. Quantify treatment effects automatically and non-invasively. IncuCyte cytotoxicity assay allows every well of a 96/384 well plate to be imaged and analyzed automatically to provide a microplate readout of cytotoxicity over time (left). Time-courses reveal concentration-dependent treatment effects (center). Transform data into concentration-response curves to compare pharmacology (right).
- Simple mix-and-read 96/384-well protocols - no washing, no fixing, no lifting
Users can plate cells by adding their treatments along with the IncuCye® cytotoxicity assay and cytotox reagent. The IncuCyte® S3 live-cell imaging and analysis system can then be used to read the plated cells kinetically. It is capable of reading up to 6 x 384-well plates simultaneously for medium/high-throughput screening.
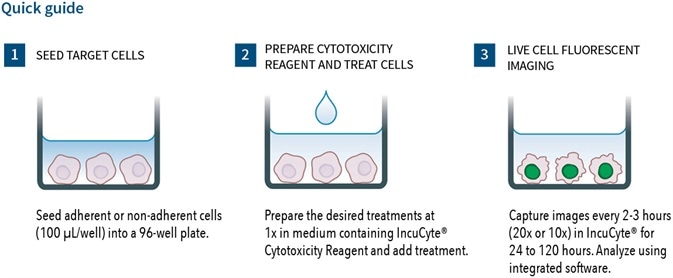
Figure 3. Adherent Cell Line Protocol.
- Multiplex with proliferation and apoptosis measurements
Multiplexed measurements of apoptosis and proliferation can be carried out when IncuCyte® cytotoxicity assays are used in combination with IncuCyte® NucLight or Caspase-3/7 reagents. Cytostatic and cytotoxic treatment effect can be readily discriminated.
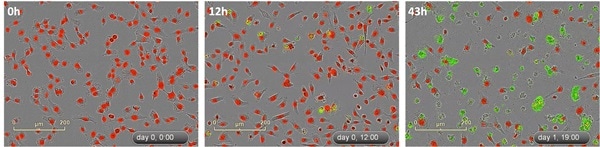
Figure 4. Multiplex cytotoxicity measurements with live-cell counting. Camptothecin (150 nM) treated HT-1080 fibrosarcoma cells (labeled with IncuCyte® NucLight Red Reagent) in the presence of the IncuCyte® Cytotox Green Reagent to detect live/dead cells over time.
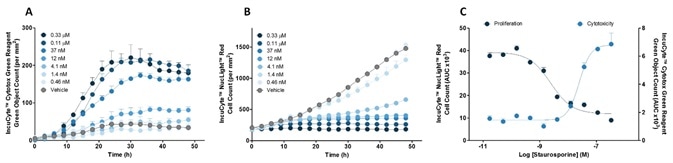
Figure 5. Quantify time-courses and concentration-dependence of cytotoxicity and proliferation. Effect of staurosporine on HT1080 cells: (A) Cytotoxicity (B) Cell Count and (C) Concentration-response curves.
 Sartorius
Sartorius
Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative Technologies Enable Medical Progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making Lab Life Easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.