The use of antibodies to treat a growing array of diseases such as neoplastic tumors, cardiovascular disease, autoimmune conditions and infectious disorders are exploding in growth because of the associated success of therapy.
Experts say that it is likely that over 70 therapeutic antibodies may be available for commercial use by 2020, yielding a revenue of $125 billion.
This growth is due to advances in antibody development and engineering, such as antibody drug conjugates, bispecific antibodies and display screening technologies. Along with a much better understanding of how molecules interact to cause human disease, these technological and scientific advances promise a new hope in the treatment of many conditions.
However, to keep the wheel rolling, novel therapeutic antibody candidates must be discovered and developed to a viable stage. This requires powerful tools which are capable of yielding results fast, with the ability to analyze multiple factors simultaneously to fully understand the ability of candidate antibodies to interrupt cellular and molecular processes leading to disease.
This article describes one such tool, the Sartorius iQue3, which enables high-throughput screening of cells and beads in suspension. Its use in multiplex screening of antibodies bound to the surfaces of cells or to antigens in peripheral blood is shown in addition its ability to perform other high content assays, to evaluate the effect of therapeutic candidates performance in multiplexed cell based and secreted protein assays.
Antibody Screening – Moving Beyond ELISA
The large size of a therapeutic antibody molecule means that it targets mostly extracellular molecules on the cell surface or in peripheral tissues.
Screening hybridomas and other antibody libraries for binding to each of these different types of target molecules has historically been conducted using ELISA, enzyme-linked immunosorbent assays, which came into use 40 years ago. These have since become a staple in this field.
The principle of ELISA is coating the wells of assay plates with a single target antigen and then adding in individual samples from a library of antibodies. When the antibody added binds to the immobilized antigen, the reaction sets off an indirect enzyme-substrate reaction which causes the color to change, facilitating the detection of the bound antibody.
Figure 1. The Sartorius iQue3 platform features easy-to-use instruments, software, and reagent kits that are optimized to work together and designed to conserve precious samples, use less reagent, and minimize time-to-answer.
ELISA technology has enjoyed popularity because of its simplicity and durability. However, it is not without its limitations, such as:
- The need to confirm specificity and rule out cross-reactivity to the antibody by following up primary screens using a single antigen with secondary and even tertiary screens using control antigens.
- Cell surface antigens are not suited to ELISA because they must be dissolved out during extraction and purified before being added to the assay plate. This can disrupt conformational epitopes which may be the primary targets for the therapeutic antibody.
- Multiple washing steps must be employed to minimize the background ‘noise’ by removing free antibody and detection reagent, which means a longer and more tedious screening process.
Multiplex High Throughput Screening for Antibody Discovery with the iQue3
As a result of these disadvantages, modern antibody laboratories are moving beyond ELISA to screening assays which cover multiple parameters and multiple antibodies at high throughputs. This increases the data collected during primary assays which mean hits can be identified based on simultaneous detection of both specificity and cross-reactivity
Having more information at this early point in the screening process increases confidence to proceed with the potential hit and increases the chance of successfully finding a candidate antibody which will proceed downstream through the discovery and development process.
The iQue3 seen in Figure 1 is a flow cytometry based, a high-throughput screening platform for suspension cells. It enables rapid high content screening of antibodies at high rates. Its features include:
- High content assays: It runs multiplex assays so that primary screens can look for binding of the antibody to both target and control antigens at the same time
- Flexibility: it can run assays to detect antibody binding to either the intact cell surface antigens or circulating antigens
- High speed: It provides a mix and read assay format for high-throughput screens
Encoding Technology – Screen Against Multiple Antigens in Every Well
The use of the Sartorius iQue3 for multiplexed screening of suspended cells and beads makes encoding technology possible. This is a term used for a high content multiplexing technique which combines different kinds of cells or beads, coated with various separate antigens to be studied, into the assay plate wells, so that many target antigens can be screened at once during the same experiment. This makes the assay more powerful and speeds up the workflow, as shown in Figure 2.
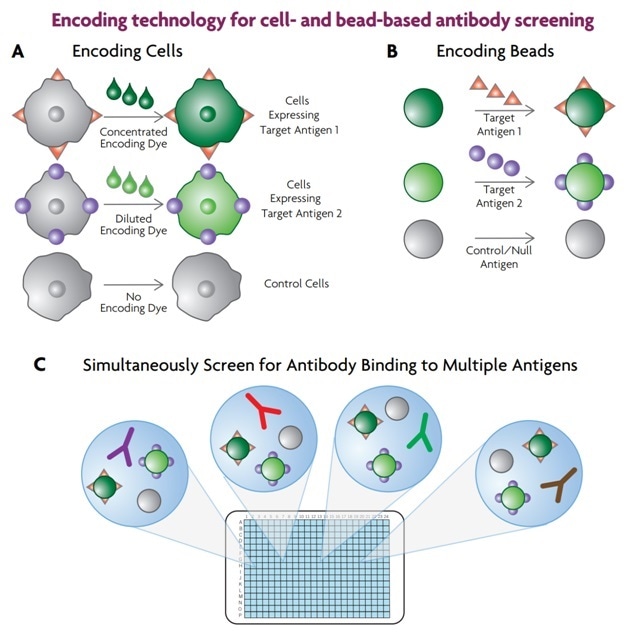
Figure 2. Cellular and soluble antigens can be multiplexed by using encoding technology. In the case of cellular antigens, cells expressing the various homologues are encoded with dye (A). Soluble antigens are attached to dye-encoded beads (B). The encoded cells or beads are combined and added to the screening plate (C) which contains the antibody library, and binding of the antibodies to the different antigens is measured.
Why Encode?
The purpose of using encoding technology is summed up as follows:
- Testing antibody binding to both test and control antigens at the same time, means each well has its own control and therefore improved accuracy of hits. The use of controls in primary screens also reduces the need for secondary and tertiary counter screens and quickens the pace of antibody screening.
- Cross reactive antibody binding can be detected because of the use of multiple antigens in the primary screens. These include families of antigen targets such as cytokines as well as related antigens from other animal species. Cross-reactivity is important when therapeutic antibodies are being developed to bind to human target antigens, and can also be used in animal models to detect efficacy and toxicity.
- Multiple antibody screens can be covered in one project, or a single library can be screened using a range of unrelated antigens. The assay plates are prepared with encoded cells or beads coated with multiple target antigens and tested using multiple libraries of antibodies, streamlining the workflow immensely.
High Throughput Screens Using Encoding Technology and the iQue3
The iQue3 has been used in situations such as the following:
Screening both Target and Control Antigens Simultaneously on Intact Cells
In the example below two cell lines were used, one expressed the control and the other the target antigen. To distinguish the two types, the target antigen-expressing cells were stained using a green fluorescent encoding dye while the cells expressing negative control antigens were left unstained.
Once the test antibody was added, the plates were incubated for 30 minutes following which anti-IgG molecules were added in the wells. These antibodies had previously been labeled with a red fluorescent dye.
After an hour of incubation the plates were read on the iQue3 and data was analyzed using the ForeCyt software as shown in Figure 3.
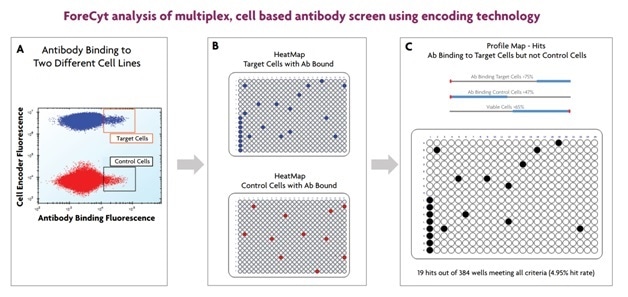
Figure 3. ForeCyt software adeptly analyzes data from multiplexed cell or bead-based screens. Encoded cells (or beads) are discriminated in one channel (y-axis; A), and binding is discriminated in another dimension (x-axis). Heatmaps for each cell line (2 in this example) show wells in which binding occurred (B). The ProfileMap feature in ForeCyt software combines data from all selected assay parameters to show which wells meet the criteria specified for each parameter (C). In this example, wells that had binding to target cells (>75%) and control cells (<47%) as well as cell viability (>65%) are considered, and only wells meeting the 3 criteria are colored.
Screening for Cross-Reactivity Using Cell-Based Assays
Figure 4 shows how Xoma Corporation researchers used multiplexed cell-based assays to detect antibodies that bound to cell surface receptors from more than one animal species. The short plate reading times, of up to five minutes, with encoding technology, meant that multiple assays could be conducted in a very short period, enabling large-scale experiments to be carried out successfully.
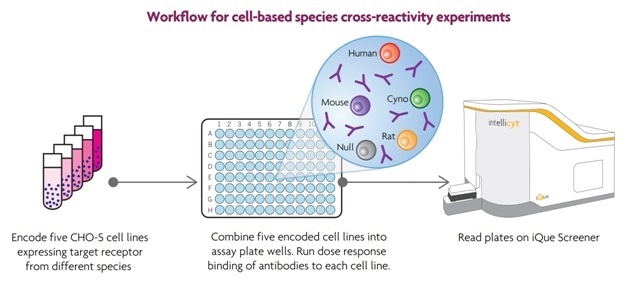
Figure 4. In these experiments, CHO cells were engineered to individually express the same target receptor from different species: human, monkey, rat and mouse (Figure 4). The parent cell line, and lines expressing the species-specific receptors were labeled separately with different concentrations of the encoding dye. The five different cell lines were combined into a single mixture, which was then distributed into wells of the assay plates. After sampling, the differentially encoded cell lines within each well were easily distinguished using ForeCyt software, which enabled the identification of cross reactive antibodies. In these experiments, each 96-well assay plate contained 12 antibodies, tested at 8 concentrations each. Each well contained five encoded cell lines.
Figure 5 shows, the binding characteristics for the different receptors to the antibodies are easily analyzed and converted to large data sets, which promotes rapid and successful discovery of novel therapeutic antibodies.
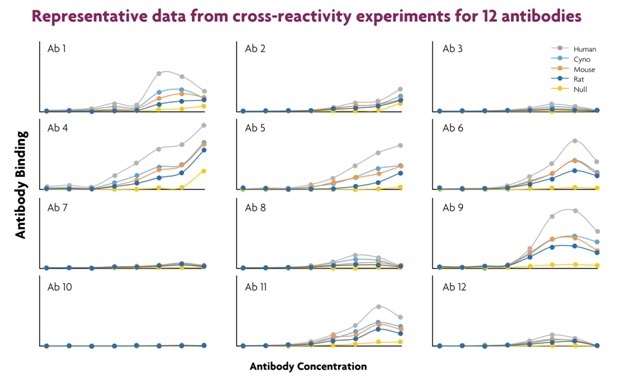
Figure 5. Cross-reactivity experiments enable the rapid discovery of antibodies that bind to both human and animal receptor homologues. Using this approach, potential drug candidates that can be used in humans and also be tested in preclinical models of efficacy and toxicity.
Screening for Cross-Reactivity Using Bead-Based Assays
Avacta Life Sciences researchers develop affimers, small binding molecules produced to be more robust than antibodies in many conditions. As Figure 6 illustrates, the work at Avacta shows how multiplexed bead based assays can be used to screen affimer libraries, generated from phage display panning outputs, for cross-reactive binders to both mouse and human immune surveillance molecules.
Compared to the typical ELISA format, the Avacta researchers were able to combine five different binding assays per well, using Sartorius technology. This reduced the protein used per target by 166 times, the number of plates required 20 times, and the total primary screen time 5 times. The whole screen was completed in below one hour.
In contrast, ELISA-based single-plex assays would need 10 plates of 384 wells or 40 plates of 96 wells each, with several milligrams of target protein of each kind.
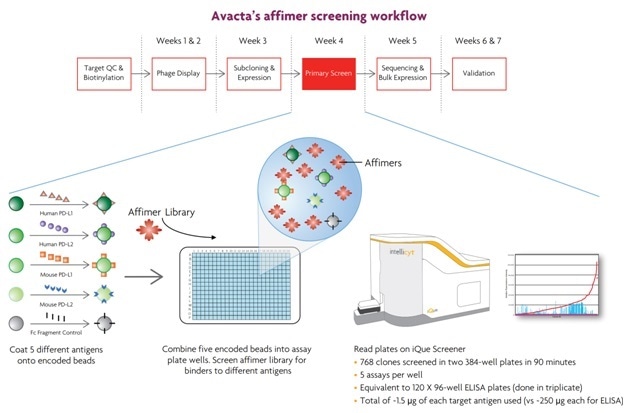
Figure 6. Encoded beads were coated with four different checkpoint modulating proteins: human PD-L1, human PD-L2, mouse PD-L1, and mouse PD-L2. Since these proteins were expressed as Fc fusions, a fifth encoded bead was coated with Fc fragment, and served as a negative control (Figure 6). The five beads were combined into a single mixture, which was then distributed into wells of 384-well assay plates. A library consisting of 768 affimers selected by panning against human PD-L1 was screened for binding to the different PD-L1 and PD-L2 antigens.
Avacta is making good use of the iQue3 platform to run multiplex assays in bead-based formats for large-scale experiments, which has led to a much smoother affimer screening workflow as shown in Figure 7.
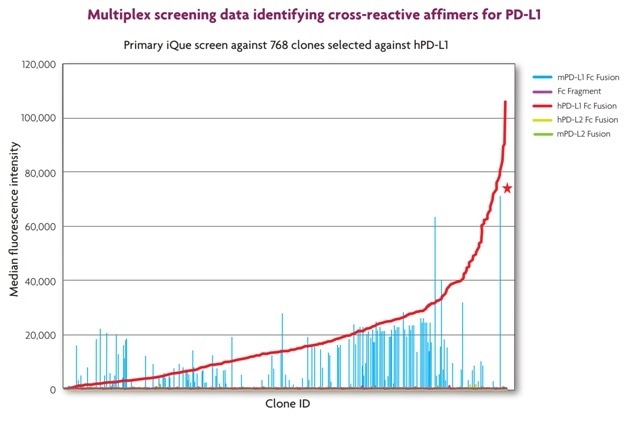
Figure 7. The screen data shows the identification of clones that bound to both human PD-L1 (red line) and mouse PD-L1 (light blue bars), but not to human or mouse PD-L2 (green and grey bars, respectively). No clones that bound to control beads coated with the Fc fragment were identified (purple bars). The star indicates one clone with high affinity for both human and mouse PD-L1.
Benefits of the iQue3 Platform
The use of the iQue3 platform has thus several advantages:
- Powerful multiplexed screening assays can be performed with encoding technology, which increase the odds of finding promising candidate antibodies and streamlining the workflow
- Decreased use of antigen
- Each well contains both control and target antigens
- Each assay can screen for cross-reactivity simultaneously
- A single screening suffices for multiple libraries
- High-throughput multiplexed screens can be performed for either cell-based or circulating antigens
- Target antigens can be coated on multiple beads to screen against antibodies targeting circulating antigens including cytokines
- Encoded cells can be used in cell-based assays to screen for antibodies that bind to multiple surface antigens present on cell surfaces of whole intact cells instead of extracted antigens, which preserves the natural epitope conformation of the antigen
- The iQue3 also offers more advantages with multiplexed antibody screens:
- Fast reading of plates, such as 96-well plates in less than 5 minutes and 384-well plates in below 20 minutes
- Simple mix and read binding assays available in no-wash format
- The ForeCyt software that comes with the iQue3 enables rapid identification of promising hits with its powerful and easy to use data analysis features
Summary
In today’s setting, therapeutic antibody development requires the use of multiplexed high-throughput screening. The Sartorius iQue3 is one such platform which has come into widespread use for high content antibody library screens.
This is due to the powerful encoding technology it uses, as well as the dramatic reduction in the use of costly antigens and reagents, the robust software analysis tool which enables quick identification of good-quality hits and the marked shortening of the time taken to discover an antibody.
References
- Chames, P., et al. (2009) “Therapeutic antibodies: successes, limitations, and hopes for the future.” British Journal of Pharmacology 157: 220-233.
- Ecker, D.M., et al. (2015) “The therapeutic monoclonal antibody market.” mAbs 7: 9-14.
- Black, C.B., et al. (2011) “Cell-Based Screening Using High-Throughput Flow Cytometry.” Assay and Drug Development Technologies 9:13-20.
 Sartorius
Sartorius
Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative Technologies Enable Medical Progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making Lab Life Easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.