Sponsored Content by SartoriusReviewed by Maria OsipovaNov 27 2023
Research suggests that publications considering microtissues and organoids provide greater predictive and translational observations than studies involving 2D monolayer cell models1,2. The use of multicellular tumor spheroids in oncology and immune-oncology research is also rising.
Specifically, researchers are accessing new functional aspects of drugs to evaluate chemoresistance and immune-tumor cell interactions. This is primarily due to the inclusion of an extracellular matrix (ECM) and additional cell types, such as stromal, endothelial, and immune cells, in the assessment of tumor microenvironment within in vitro models.
Evaluating tumor spheroid growth and shrinkage using existing techniques is constrained by time-consuming, costly, and complex experimental procedures. Methods currently involve fluorescent probes disrupting biological processes, single timepoint analysis that may overlook important temporal details, or indirect biochemical measurements failing to capture valuable morphological cell information.
To address these shortcomings, the Incucyte® Live-Cell Analysis System provides an automated phase contrast and two-color fluorescence image acquisition within a standard cell culture incubator that optimizes cell viability and maintenance of physiological relevance.
The Incucyte® Live-Cell Analysis System can scan tissue culture samples repeatedly over pre-determined intervals, allowing users to monitor cultures and generate quantifiable, kinetic information.
The platform is coupled with the Incucyte® Spheroid software, which provides rapid, flexible, and powerful control for the analysis of live cells.
Analytical methods include the acquisition of images and the processing and visualization of data. The reagents used for the analysis include non-invasive cell labeling, nuclear-targeted green fluorescent protein (GFP), and red fluorescent protein (RFP) for cell quantification.
Principle of the assay
This article examines how to study the growth of 3D tumor multi-spheroids in co-culture with fibroblasts or immune cells using the Incucyte® Live-Cell Analysis System and Incucyte® 3D Multi-Tumor Spheroid Assays.
The monitoring ability of the platform captures multiple time points while the depth of focus Brightfield (DF Brightfield) image acquisition provides long-term imaging of multiple tumor spheroids grown on an ECM bed (Matrigel™).
Built-in Incucyte® processing definitions can further improve image acquisition results with the ability to mask high-contrast images. Object characteristics such as size, count, and eccentricity can also be plotted automatically over time to determine spheroid formation and growth rates. The platform's capacity enables up to six 96-well plates to run in parallel and analyze thousands of images.
The following sections describe the data and validation methods demonstrating the effectiveness of the Incucyte® Live-Cell Analysis System. In particular, the system is used to kinetically visualize and quantify the effect of stromal cells on tumor multi-spheroid morphology and the sensitivity to chemotherapeutic agents, while assessing immune cell-mediated toxicity within tumor multi-spheroids.
Material and methods
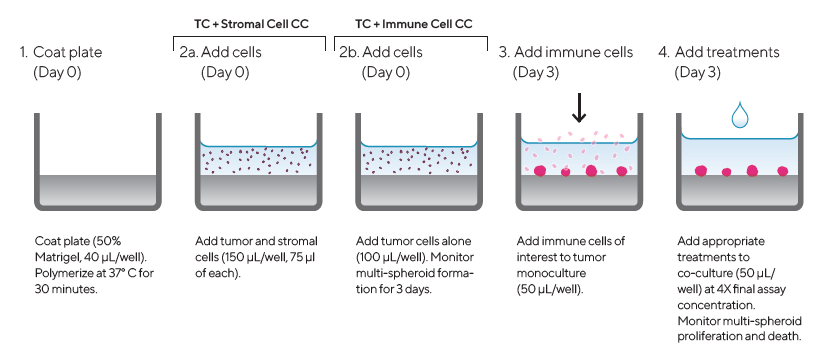
Figure 1. Assay Workflow. Image Credit: Sartorius
1. Coat 96-well micro-titer plate with a Matrigel™ layer (Corning) (40 μL/well, diluted in serum-free media to a minimum concentration of 4.5 mg/mL) then polymerized at 37 °C for 30 min
2. Harvest, count, and seed cells of interest into pre-coated 96-well plates at target densities (150 or 100 μL/well)
a. Seed tumor and stromal cells at 1:1 ratio (150 μL/well, 75 μL of each cell type) in tumor and stromal cell co-culture
b. Seed tumor cells alone in tumor and immune cell co-culture (100 μL/well)
3. Monitor multi-spheroid formation for three days using an Incucyte® Image Analysis system (DF® Brightfield acquisition, 10X magnification, every six hours)
4. Once the multi-spheroid has formed, add immune cells of interest to tumor monoculture at an optimized Effector-to-Target ratio (E:T) in a volume of 50 μL/well
5. Add appropriate treatments to co-culture assay plates (50 μL/well) at 4X final assay concentration to achieve a final volume of 200 μL/well
6. Multi-spheroid proliferation monitored in Incucyte® S3 (6-hour repeat scanning) for up to 10 days
All cell culture reagents were sourced from Life Technologies, except where specified. MDA-MB-231 (ATCC), SK-BR-3 (ATCC), and SKOV3 (EACC) cell cultures underwent stable transfection using Incucyte® Nuclight Red Lentivirus Reagent (EF1 Alpha Promoter, Puromycin selection, Cat. No. 4625), prepared following the Essen BioScience protocol.
For BT-474 (ATCC) cells, they were stably transfected with Incucyte® Cytolight Green Lentivirus Reagent (EF1 Alpha Promoter, Puromycin selection, Cat. No. 4481), following the Essen BioScience protocol.
Lapatinib and Camptothecin were procured from Sigma, while ZK164015 was obtained from Tocris Bioscience.
Normal human dermal fibroblasts (NHDFs) were acquired from CalTag Medsystems (Cat. No. ZHC-5102), and MCF7 Nuclight Red cells were sourced from Essen BioScience (Cat. No. 4524).
All cell lines were cultured in MEM medium supplemented with 10% FBS, 1% sodium pyruvate, and 1% non-essential amino acids. They were grown to confluence in 75 cm2 tissue culture-treated flasks (Corning). Subsequently, cells were harvested and seeded into 96-well plates (Corning No. 3595) coated with a 40 μL/well Matrigel base layer. This allowed for the formation of multi-spheroids of the desired size three days after cell seeding.
Spheroid development was monitored using an Incucyte® S3 system over three days at 6-hour intervals. Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats from healthy donors using Lymphoprep™ density gradient medium from StemCell Technologies and were maintained in RPMI 1640 supplemented with 10% heat-inactivated FBS.
Influence of stromal cells on multi-spheroid morphology
Following the procedure, Normal Human Dermal Fibroblasts (NHDFs) had an observable effect on tumor multi-spheroid morphology. SK-BR-3 cells were seeded alone or with NHDFs (1:1 ratio, 1,000 cells/well for each) and DF Brightfield images were taken every six hours.
Monocultured SK-BR-3 cells did not form compact multi-spheroids, whereas co-cultures with NHDFs did form more compact aggregates (Figure 2). The Incucyte® Spheroid Software Module kinetically quantified both morphologies, using size measurements to track multi-spheroid growth (Total BF Area).
Various temporal growth profiles were measured through live-cell analysis for a panel of four breast tumor multi-spheroids (MCF7, MDA-MB-231, SK-BR-3, and BT-474) co-cultured with NHDFs (Figure 2 time-course plot).
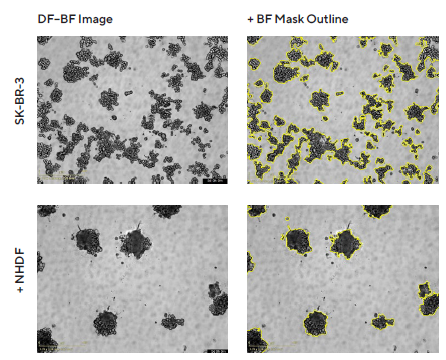
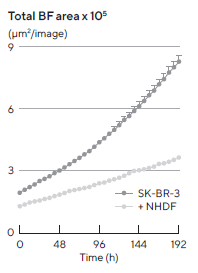
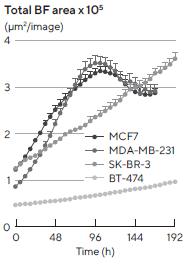 Figure 2. Morphology observations with Incucyte® DF-BF images and quantification of multi-spheroid size and kinetic growth using real-time analysis. SK-BR-3 cells were seeded in flat bottom, 96-well plates on a bed of Matrigel in mono- or co-culture with NHDFs (1:1 ratio, 1,000 cells/well for each) and multi-spheroids (MS) allowed to form (3 d). Incucyte® extended depth of focus Brightfield (DF Brightfield) images (8 d post cell seeding) of SK-BR-3 MS in mono- or co-culture with NHDFs. Brightfield outline mask shown in yellow. Note, the influence of NHDFs on SK-BR-3 MS morphology and size (Total Area). Time course plots show the individual well Total Brightfield Object Area (μm2) (y-axis) over time (h) (x-axis) and illustrate cell type-specific kinetic growth profiles for a range of breast tumor MS co-cultured with NHDFs. Data were collected over 192 h period at 6 h intervals. Each data point represents mean ± SEM, n=15 wells. Image Credit: Sartorius
Figure 2. Morphology observations with Incucyte® DF-BF images and quantification of multi-spheroid size and kinetic growth using real-time analysis. SK-BR-3 cells were seeded in flat bottom, 96-well plates on a bed of Matrigel in mono- or co-culture with NHDFs (1:1 ratio, 1,000 cells/well for each) and multi-spheroids (MS) allowed to form (3 d). Incucyte® extended depth of focus Brightfield (DF Brightfield) images (8 d post cell seeding) of SK-BR-3 MS in mono- or co-culture with NHDFs. Brightfield outline mask shown in yellow. Note, the influence of NHDFs on SK-BR-3 MS morphology and size (Total Area). Time course plots show the individual well Total Brightfield Object Area (μm2) (y-axis) over time (h) (x-axis) and illustrate cell type-specific kinetic growth profiles for a range of breast tumor MS co-cultured with NHDFs. Data were collected over 192 h period at 6 h intervals. Each data point represents mean ± SEM, n=15 wells. Image Credit: Sartorius
However, growth profiles demonstrated a time-dependent effect on spheroid morphology when NHDFs were co-cultured with MDA-MB-231 cells (1:1 ratio, 1,000 cells/well for each). Specifically, MDA-MB-231 multi-spheroids transitioned from a stellate, branched appearance early in development to a clustered round appearance after six days (Figure 3).
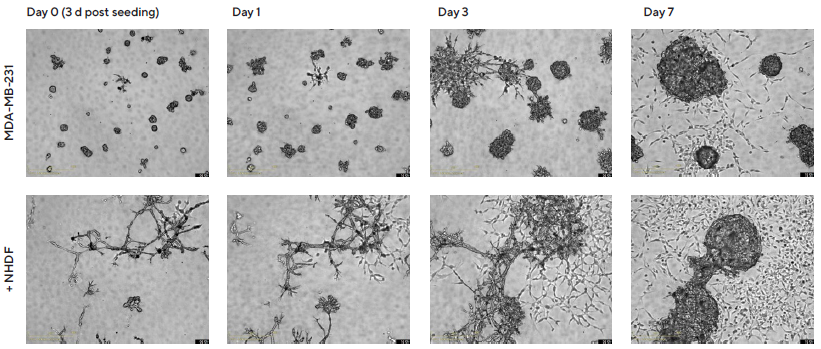
Figure 3. Temporal effects on morphology revealed through Incucyte® DF Brightfield imaging. MDA-MB-231 cells were seeded in flat bottom 96-well plates on a bed of Matrigel in mono- or co-culture with NHDFs (1:1 ratio, 1,000 cells/well for each) and multi-spheroids allowed to form (3 d). Incucyte® S3 DF Brightfield images compare mono- and co-culture conditions over 7 d (10 d post cell seeding). Note the temporal impact of NHDFs on spheroid morphology. Image Credit: Sartorius
Pharmacological analysis using a 96-well multi-spheroid growth and shrinkage assay
To demonstrate the suitability of this 3D tumor and stromal cell co-culture for assessing compound toxicity, a pharmacological investigation was conducted using four commonly used breast tumor cell lines: MCF7, MDA-MB-231, SK-BR-3, and BT-474.
The cells were co-cultured with NHDFs at a ratio of 1:1, with 1,000 cells of each type per well.
After allowing the multi-spheroids to form for three days, treatment was initiated and continued for up to 10 days using standard-of-care and cytotoxic agents, namely Lapatinib, ZK164015, and Camptothecin (CMP).
The real-time, automated views from Incucyte® enabled the observation of treatment effects on multi-spheroid size (Total BF Area).
A concentration-dependent inhibition of ZK164015 and CMP on spheroid size was observed from the 96-well vessel views of MCF7 multi-spheroids with overlay analysis mask and treatment time courses (Figure 4).
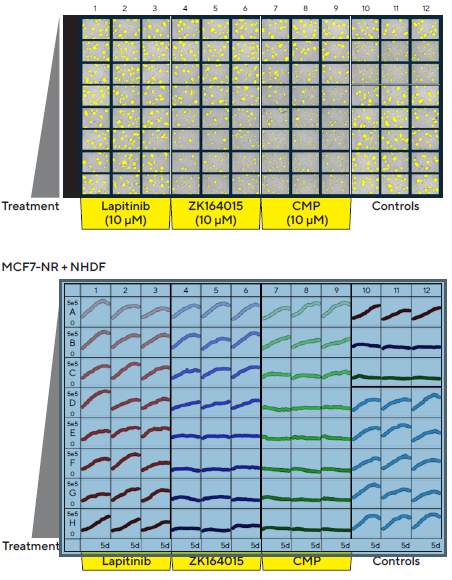
Figure 4. Incucyte® S3 vessel views enable rapid visualization and quantification of treatment effects. MCF7 cells co-cultured with NHDFs were seeded in pre-coated (Matrigel) flat bottom 96-well plates (1:1 ratio, 1,000 cells/well of each) and multi-spheroids allowed to form (3 d). Spheroids were then treated with serial dilutions of known standard of care and cytotoxic compounds (5 d). Incucyte® microplate vessel views show effects of treatments on multi-spheroid size. Top image shows Brightfield Object Area (μm2) segmentation mask (yellow) 5 d post treatment. Bottom image shows the individual well Total Brightfield Object Area (μm2) (y-axis) over time (0–5 d post treatment) (x-axis). Image Credit: Sartorius
As a result, findings from brightfield images indicate the cytotoxic effects of Lapatinib on SK-BR-3 size and a non-specific cytotoxic effect of CMP on both MCF7 and SK-BR-3 spheroids (Figure 5).
Area under curve (AUC) analysis over the time-course data obtained showed a concentration-response curve (Figure 6). Data showed that Lapatinib and ZK164015 inhibited multi-spheroid growth, aligning with the known expression profile of receptors targeted by these agents.3
While Lapatinib, which is the dual EGRF and HER2 tyrosine kinase inhibitor, led to a concentration-dependent inhibition of SK-BR-3 and BT-474 multi-spheroid growth, the estrogen receptor (ER) antagonist ZK164015 was an inhibited MCF7 multi-spheroid growth. In contrast, ZK164015 had limited effects on multi-spheroids devoid of ER expression. The DNA topoisomerase inhibitor, CMP, caused similar inhibition across all multi-spheroid types.
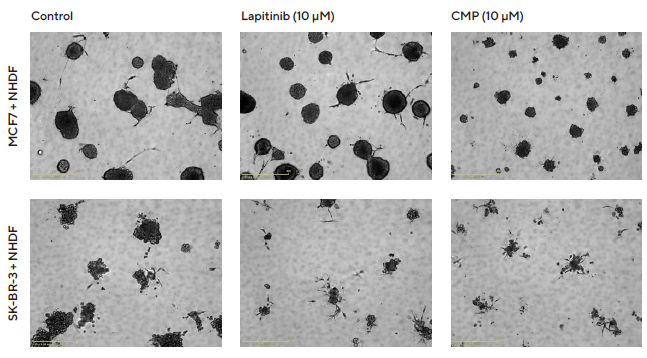
Figure 5. Interrogation of images to gain greater insight. MCF7 or SK-BR-3 cells co-cultured with NHDFs were seeded in pre-coated (Matrigel) flat bottom 96-well plates (1:1 ratio, 1,000 cells/well of each) and multi-spheroids allowed to form (3 d) prior to treatment with Lapatinib and Camptothecin (CMP). Incucyte® Brightfield images (5 d) show the effects of treatments on multi-spheroid size and integrity. Note: cytotoxic effects of CMP on both MCF7 and SK-BR-3 multi-spheroids and their difference in sensitivity to Lapatinib. Image Credit: Sartorius
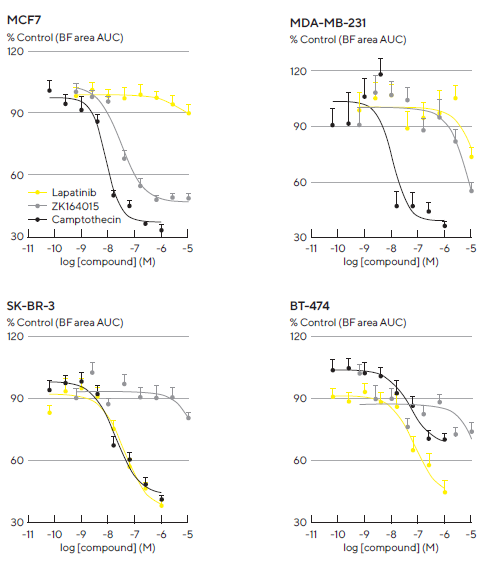
Figure 6. Robust and reproducible pharmacological analysis through generation of concentration response curves. A panel of four tumor breast cell lines were seeded in 96-well flat bottom plates with NHDFs (1:1 ratio, 1,000 cells/well for each on a bed of Matrigel). Multi-spheroids were allowed to form for 3 d prior to treatment with standard-of-care and cytotoxic agents. Concentration response curves (CRCs) represent the area under curve (AUC) of the Total Brightfield Area (μm2) time course data (not shown) from 0–6 d (MCF7, MDA-MB-231) or 0–10 d (SK-BR-3, BT-474) post-treatment. Each data point represents mean ± SEM, n=9–12 wells. Note: differential compound pharmacology across cell types. Image Credit: Sartorius
Herceptin®-induced ADCC in HER2-positive multi-spheroids
A key mechanism in immunotherapy treatments is the regulation of immune-mediated killing by mono-clonal antibodies4.
Antibody-dependent, cell-mediated cytotoxicity (ADCC) is induced by the binding of antibodies, such as Herceptin (a humanized monoclonal antibody targeting HER2 receptor), to receptors.
Therefore, HER2-positive (SKOV3 and BT474) and HER2-negative (MCF7) multi-spheroids were used to examine the impact of Herceptin-induced ADCC.
Multi-spheroids of tumors that either expressed nuclear-restricted RFP (NR) or cytoplasmic GFP (CyG), were co-cultured with isolated peripheral blood mononuclear cells (PBMCs) (E:T, 5:1) before being treated with Herceptin.
Fluorescence intensity from within the brightfield boundary was then used to quantify multi-spheroid proliferation and immune cell-mediated cytotoxicity. When alone, PBMCs or Herceptin had limited effects on multi-spheroid size or viability (fluorescence intensity, Figure 7). However, when combined, Herceptin-activated PBMCs, lead to a loss of MS viability.
Due to Herceptin's action, an effect that caused cytotoxicity, dependent on concentration, was observed in HER2-positive multi-spheroids but not in HER2-negative multi-spheroids.
BT-474 multi-spheroids, in particular, were found to be the most susceptible targets, exhibiting the highest concentration of Herceptin (1 μg/mL) and achieving approximately 80% inhibition, compared to approximately 50% in SKOV3 multi-spheroids (Figure 8, concentration-response curve).
Activation of the T cell population in PBMCs was achieved by an AntiCD3 antibody and IL-2 cocktail (10 ng/mL each), resulting in maximal cell killing across all cell types (Figure 8, time-courses).
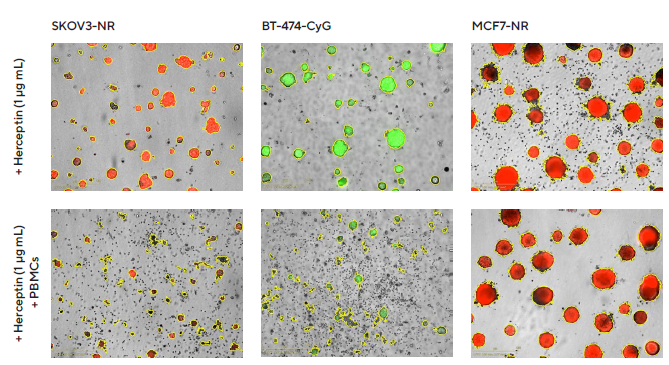
Figure 7. Impact of Herceptin-induced PBMCs on multi-spheroid proliferation. Tumor cells either stably expressing nuclear restricted RFP (SKOV3-NR, MCF7-NR) or cytoplasmically restricted GFP (BT-474-CyG) were seeded in flat bottom 96-well plates (1,000 cells/well on a bed of Matrigel). Multi-spheroids were allowed to form (3 d) prior to addition of freshly isolated PBMCs (E:T, 5:1) and Herceptin. Incucyte® S3 brightfield and fluorescence images (7 d; SKOV3-NR, MCF-NR or 10 d; BT-474-CyG) compare the effect of Herceptin on spheroid proliferation in absence (top panel) and presence (bottom panel) of PBMCs (Brightfield outline mask shown in yellow). Note the loss of fluorescence intensity in HER2- positive (SKOV3 and BT474) multi-spheroids in the presence of PBMCs. Image Credit: Sartorius
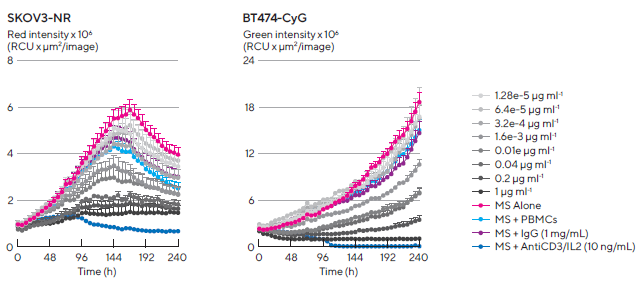
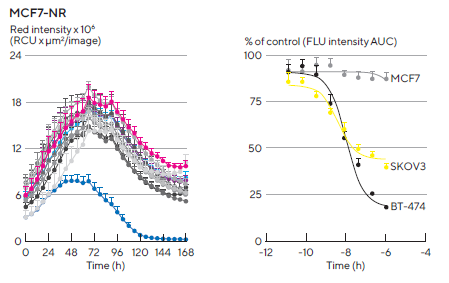
Figure 8. Kinetic quantification of ADCC immune cell killing of HER-2 positive multi-spheroids. Tumor cells were seeded in flat bottom 96-well plates (1,000 cells/well on a bed of Matrigel) and allowed to form multi-spheroids (MS) for 3 d. Once formed, MS were co-cultured with freshly isolated PBMCs (E:T, 5:1) and treated with serial dilutions of Herceptin. Time-courses show multi-spheroid death quantified as a loss of fluorescence intensity within the spheroid brightfield object. Concentration-response curves to Herceptin show sensitivity differences between HER2-positive multi-spheroids (SKOV3 and BT-474). Treatments targeting T cell populations (Anti-CD3 and IL-2, 10 ng/mL) induced maximal MS cytotoxicity across all cell types. Data were collected over 10 d at 6 h intervals. Each data point represents mean ± SEM, n=4 wells. Image Credit: Sartorius
Conclusions
The effectiveness of the Incucyte® Live-Cell Analysis System, together with the Incucyte® Spheroid Software Module, is demonstrated through the analysis of 3D multi-spheroid co-cultures with stromal or immune cells over time and is applicable to compound testing. It has been shown that:
- The live-cell analysis found that SK-BR-3 formed more compact multi-spheroids when cultured with NHDFs
- Live-cell imaging revealed temporal effects of NHDFs on MDA-MB-231 multi-spheroid morphology
- Cell type-specific temporal growth profiles were determined for a panel of breast tumor multi-spheroids
- The capability to perform real-time compound profiling in a 96-well format
- The ability to kinetically visualize and quantify antibody-dependent cell-mediated cytotoxicity (ADCC) immune cell-induced within target tumor multi-spheroids in real-time
- Herceptin-activated PBMCs caused a concentration-dependent loss of HER2-positive multi-spheroid viability
Incorporating an ECM and additional cell types (e.g. stromal cells or immune cells) into 3D models may provide more relevant translational models for the study of the tumor microenvironment on tumor biology.
Features of the Incucyte® Live-Cell Analysis System are advantageous for monitoring and quantifying 3D multi-spheroid biology in real time. This includes the DF Brightfield imaging, which allows label-free study of 3D spheroid morphology and proliferation in 96-well assay formats for enhanced throughput.
Advantages also include the ability to run without predefined endpoint, consistent segmentation and quantification of brightfield images, which enables kinetic assessment of cell-dependent growth profiles, and the impact of stromal cells on tumor multi-spheroid resistance to chemotherapeutic agents.
Using brightfield with fluorescence imaging enables visualization and quantification of immune cell-mediated toxicity of tumor multi-spheroids.
Incucyte®’s automated image acquisition, combined with user-friendly analysis tools and lab-tested protocols, allows non-expert users to quickly generate reproducible data, perform analysis, and generate publication-ready graphics. Taken together, the Incucyte® Live-Cell Analysis System, Spheroid Software Module and reagents provide a unique and efficient technical platform that can be incorporated into existing workflows.
Acknowledgments
Produced from materials originally authored by Kalpana Patel, Miniver Oliver, Nevine Holtz, Tim Jackson, Nicholas Dana, Tim Dale, Del Trezise from Sartorius.
References and further reading
- Lv D, et al. Three-dimensional Cell Culture: A Powerful Tool in Tumor Research and Drug Discovery (Review). Spandidos Publications. Oncology Letters, 14;6999–7010 (2017)
- Verjans ET, et al. Three-dimensional cell culture models for anticancer drug screening: Worth the effort? Cell Physiol. Apr;233(4);2993–3003 (2018)
- Subick K, et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer: Basic and Clinical Research, 4;35–41 (2010)
- Griggs J, et al. The State of the Art: Immune-mediated Mechanisms of Monoclonal Antibodies in Cancer Therapy. British Journal of Cancer, 104;1807–1812 (2009)
About Sartorius
Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative technologies enable medical progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making lab life easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.