Sponsored Content by SartoriusReviewed by Maria OsipovaFeb 19 2026
Jasmine Trigg, Kirsty McBain, Daryl Cole, Nicola Bevan
Sartorius UK, Royston, Hertfordshire, UK
Cell signaling pathways contain a wide range of secreted proteins and cytokines, and correctly quantifying these molecules is critical for basic research and therapeutic development.
The role of the nervous-immune interaction during inflammation and neurodegenerative illness has become increasingly acknowledged in recent years.
Research is now focusing on improving our understanding of cytokines involved in disease development and progression, with potential therapeutic targets emerging for neuroinflammatory and neurodegenerative disorders.
Recent studies have demonstrated how iQue Qbeads® and High-Throughput Screening (HTS) by Cytometry can be used to simultaneously analyze a wide range of released proteins in physiologically relevant immune cell or iPSC-derived neuroinflammatory models.
Introduction
Cellular signaling pathways that control cell-to-cell contact include a wide range of secreted proteins and cytokines.
Accurately quantifying the secretion or aberrant synthesis of these molecules is central to fundamental research and therapeutic development, improving our understanding of physiological processes, such as immune cell activation, and faulty cellular signaling implicated in almost all diseases.1
The relevance of the nervous-immune interface in inflammation and neurodegenerative disorders is increasingly recognized.
Recent studies have demonstrated that inflammation not only plays a critical role in the development of neurodegeneration but can also act as an initiator, with inflammatory signaling exerting both beneficial and deleterious effects depending on the stage of the disease.2,3
A more comprehensive understanding of the timing, cell specificity, and molecular mechanisms, as well as the use of more translational cellular models, such as iPSC-derived, will be critical for developing therapeutic strategies that block or enhance inflammatory signaling pathways involved in neurodegenerative diseases.
Over the last decade, there has been a considerable surge in research focused on the measurement and study of cytokines. These investigations focus on the ability to detect, quantify, and distinguish multiple secreted factors from a complex mixture of biomolecules in a given sample.4
A variety of recognized immunoassay methods, including enzyme-linked immunosorbent assays (ELISA) and flow cytometry-based tests, are now used. However, they have significant disadvantages: low throughput, arduous and time-consuming processing, often requiring multiple rounds of optimization, labeling, extended incubations, and repeated washes.5
iQue Qbeads® trap specific proteins on different types of beads and enable multiplexed quantification of a wide range of cytokines, adhesion molecules, enzymes, and growth receptors.
These ready-to-run kits were designed for use on the iQue® High-Throughput Screening (HTS) Cytometry Platform and offer an integrated solution with the advantages of simple no-wash protocols, high assay miniaturization, fast sampling speeds, and lower costs.
Here, we observe how a combination of pre-built or bespoke Qbead® kits and HTS by Cytometry can be used to quantify various secreted proteins in inflammatory and neuroinflammatory models.
This adaptable methodology can also be used to study iPSC-derived neuronal models, where nonperturbing methods and small sample volumes are required to temporally monitor inflammatory protein release in sensitive and valuable cell types.
Results
Secreted protein release in inflammatory models of Akt activity
LPS stimulation of immune cells activates cells via the release of pro-inflammatory cytokines, a process regulated by the Akt pathway.9,10 Inhibition of this pathway, either directly targeting Akt or upstream PI3K, has been shown to reduce Akt phosphorylation and inflammatory cytokine release.11
Dysregulation of the Akt pathway has been linked to a variety of diseases, including cancer, inflammatory ailments, and neurodegenerative disorders.12,13 Therefore, targeting the Akt pathway may provide therapeutic prospects for these problems.
To explore immune cell activation, a custom iQue Qbeads® kit was created using the assay builder to quantify 11 inflammatory cytokines and chemokines of interest in mice (Figure 2).
Murine RAW264.7 monocytes were serum fasted for three hours to inactivate Akt before being treated with several concentrations of LPS (1.56 - 100 ng/mL) in the absence or presence of MK2206, an allosteric Akt inhibitor.
Supernatant samples were obtained and examined four hours after stimulation. Each secreted protein was identified using a pre-gated template provided with the kit (Figure 2A).
Protein concentrations in samples were determined using standard curves, with an example curve for CCL3 (MIP-1α) (Figure 2B). The heatmap function allows for quick visualization of secreted protein release (Figure 2C).
The study found that LPS caused a concentration-dependent increase in the release of inflammatory proteins CCL3 (Figure 2D) and TNFα (data not shown), but little to no release of other cytokines and chemokines of interest.
LPS stimulation with MK2206 reduced CCL3 release compared to LPS alone, with values of 2,174 ± 330 and 3,026 ± 209 pg/mL, respectively (Figure 2E).
The study also found that MK2206 alone reduced baseline CCL3 release compared with the vehicle; these findings suggest that the Akt pathway plays a role in the release of inflammatory proteins.
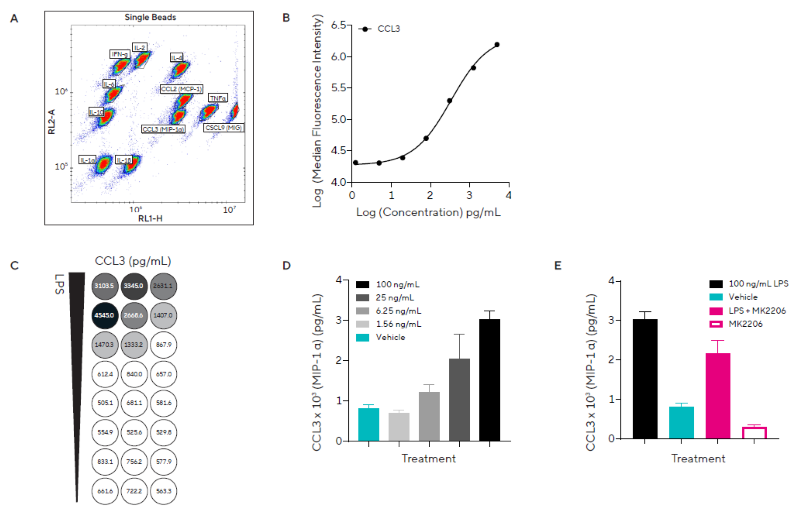
Figure 2. Pro-inflammatory Stimulation Induces Concentration-Dependent Release of Inflammatory Cytokines. A) Dot plot showing single beads and Forecyt® gating strategy for the custom murine Qbead panel designed to assess 11 inflammatory secreted proteins of interest. B) Standard curve from which CCL3 (MIP-1α) protein concentrations were derived. C) Heatmap for CCL3 concentration. D) CCL3 concentration in response to a concentration range of LPS or vehicle. E) CCL3 concentration in response to a single concentration of LPS (100 ng/mL) in the presence of direct Akt inhibitor MK2206 (5 μM). Data presented as mean + SEM at 4 hours post-stimulation, n = 3 replicates. Image Credit: Sartorius
Using HTS cytometry to assess phenotypic differences following macrophage polarization
Macrophages are important immune system components that can be triggered into the M1 or M2 phenotypes in response to environmental cues. M1 macrophages, triggered by LPS and IFNγ, have a proinflammatory phenotype and amoeboid shape.
M2 macrophages, on the other hand, are triggered in two ways and can be classified into at least four categories depending on the stimuli used. IL-4 induces M2a macrophages, which have an anti-inflammatory phenotype with a mixture of ramified and amoeboid morphologies.14,15
Macrophages can occur in varied populations, and multiparametric techniques are required for their characterization, including cell shape, receptor expression profiles, and pro-inflammatory factor secretion.16
The researchers studied secreted protein release from human primary monocytes differentiated into M0, M1, or M2a macrophages over a 7-day period (Figure 3). Live-cell analysis was used to track morphological changes during cell differentiation.
Supernatant samples collected on Days 3, 6, and 7 of differentiation were analyzed using HTS by Cytometry and the pre-built Human Inflammation Panel Kit, which measures 7 human cytokines and chemokines involved in inflammatory responses to disease conditions.
Representative phase-contrast images of morphological changes associated with M1 and M2a phenotypes are displayed, with amoeboid and ramified morphologies detected, respectively (Figure 3A).
On Day 7, an increase in the release of five secreted proteins was observed in M1 macrophages relative to M0 and M2a macrophages (Figure 3B), with little to no IL-2 release in any phenotype.
On Day 7, M2a macrophages produced somewhat less CCL2 than M0 macrophages (Figure 3C). These results are consistent with the pro-inflammatory nature of M1 macrophages.
Overall, the data shows that macrophages have successfully polarized into pro-inflammatory M1 and anti-inflammatory M2a phenotypes. These are differentiated by their unique morphologies and patterns of proinflammatory secreted protein production.
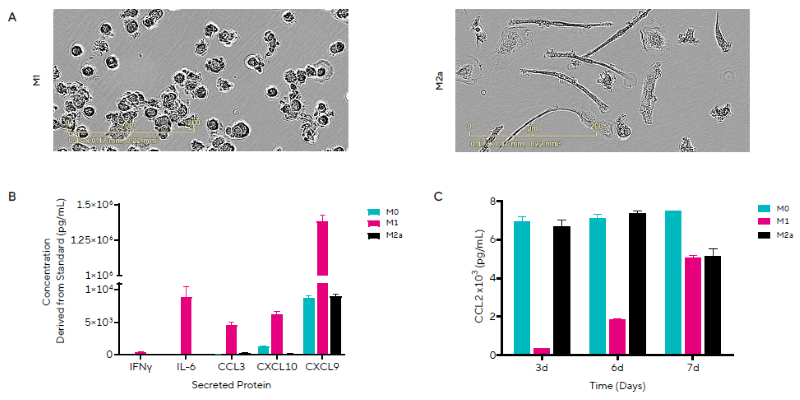
Figure 3. M1 Macrophage Polarization Induces Inflammatory Secreted Protein Release. Representative images on Day 7 of macrophage morphology following polarization to an M1 or M2a phenotype. B) Concentrations of 5 out of the 7 secreted proteins are shown for each macrophage phenotype on Day 7. C) Temporal profile of CCL2 (MCP-1) concentrations for each macrophage phenotype. Data presented as mean + SEM, n = 3 replicates. Image Credit: Sartorius
Neuroinflammatory protein release in healthy and diseased iPSC-derived models
Human iPSC-derived models are increasingly being employed for in vitro research into the human central nervous system and its interactions with immune systems.
These models allow for the generation of healthy and disease-specific neurons and support cells, such as microglia, creating a more translational system for studying human development and disease, as well as the study of emerging therapeutics for neuroinflammatory and neurodegenerative conditions.17
Because of the inherent cell and model sensitivity, non-invasive and cell-sparing methods are required to reliably monitor and characterize these complex models.
Microglia, the brain's resident macrophages, play an important role in neuroinflammatory reactions, exhibiting either proinflammatory (M1) or anti-inflammatory phenotypes.18 They have a vital role in the genesis and progression of neurodegenerative diseases.19,20
This study investigated the effects of pro-inflammatory stimuli on iPSC-derived microglia. In Figure 4, the researchers used a combination of IFNγ and LPS as a positive control to induce an M1 pro-inflammatory microglial phenotype.
Microglia were treated with varying concentrations of these stimuli or a single concentration (100 ng/mL) in the presence of the anti-inflammatory compound DEX (500 nM), and supernatant samples collected at 4, 24, or 48 hours after treatment were analyzed using HTS by Cytometry and the Human Inflammation Panel Kit.
Figure 4A shows the gating strategy used to identify the released proteins, whereas Figure 4B shows example curves for the standards used to calculate protein concentrations in the samples. IFNγ was solely evaluated in cases where it was not administered as a stimulant.
The researchers observed the secretion of multiple proteins in response to both stimulants. Following IFNγ stimulation, CXCL10 (IP-10) was produced in a temporal and concentration-dependent manner, with levels marginally lower than the positive control. DEX did not prevent this rise (Figure 4C).
Similar temporal release of CCL3 (MIP-1α) was detected, with a minor concentration-dependent effect that was reduced in the presence of DEX (Figure 4D).
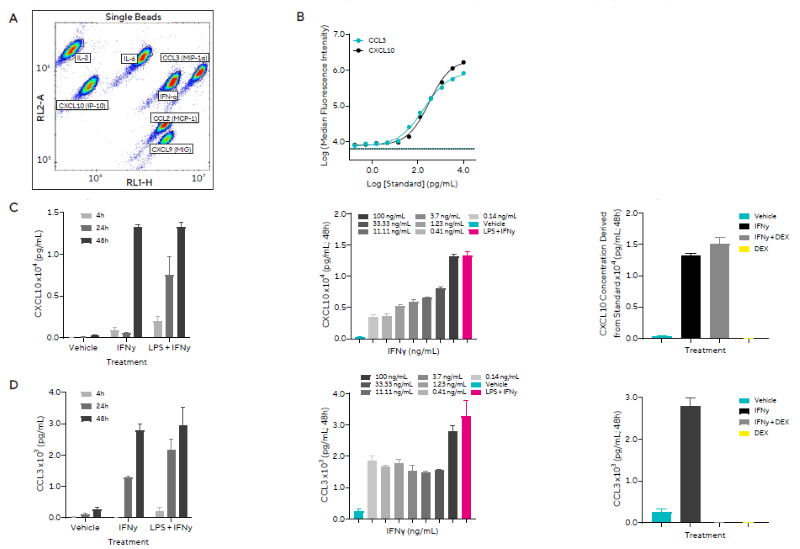
Figure 4. Pro-inflammatory stimulation of microglia induces the release of inflammatory secreted proteins. Human iPSC-derived microglia were treated with LPS and IFNγ, in the absence or presence of DEX. A) Forecyt® gating strategy for each secreted protein. B) Cytokine standards from which concentrations were derived are shown for two of the seven secreted proteins, CCL3 and CXCL10. C) CXCL10 concentration in response to 100 ng/mL IFNγ or positive control across 4 to 48 hours (left), a concentration range of IFNγ at 48 hours (middle), and 100 ng/mL IFNγ in the presence or absence of DEX at 48 hours (right). D) CCL3 (MIP-1α) concentration in response to 100 ng/mL IFNγ or positive control across 4 – 48 hours (left), a concentration range of IFNγ at 48 hours (middle), and 100 ng/mL IFNγ in the presence or absence of DEX at 48 hours (right). Data presented as mean + SEM, n = 3 replicates. Image Credit: Sartorius
In a second study on neuroinflammation and neurodegeneration, the researchers measured secreted protein levels in healthy or AD iPSC-derived models and examined neurons in mono- or co-culture with healthy iPSC-derived microglia.
Supernatant samples collected on Day 30 were analyzed by HTS using Cytometry and a custom-built kit designed to detect the release of eight human secreted proteins of interest (Figure 5).
Overall, it was found that neurons in co-culture produced more GM-CSF and CCL2 than neurons in mono-culture, suggesting that cultures with microglia exhibit distinct baseline secretory profiles.
In addition, the findings demonstrated that levels of three inflammatory cytokines, IL-6, CSF-2, and CCL2, were consistently higher in diseased than in healthy phenotypes in co-culture settings.
CCL2, a chemokine linked to microglia expansion in neuroinflammatory regions and altered amyloid-beta metabolism in AD21, rose from 3,197 ± 297 to 6,235 ± 628 pg/mL in healthy versus diseased co-cultures, respectively.
This method enables high-throughput multiplexed cytometry, with the assay's downsizing allowing temporal monitoring of secreted protein release in a non-invasive way. Finally, this allows the researchers to track sensitive translational cellular models and delve deeper into neuroinflammatory pathways and potential therapeutic benefits.
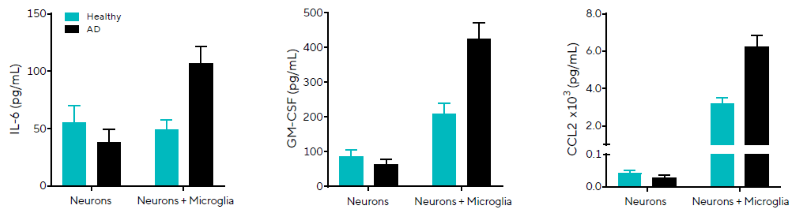
Figure 5. Elevated pro-inflammatory secreted proteins observed in AD iPSC-derived neuron and microglia co-cultures. Healthy or AD (PSEN1 mutation) neurons co-cultured on Day 19 with iPSC-derived monocytes. IL-6, GM-CSF, and CCL2 concentrations derived from standards are shown for healthy or AD iPSC-derived mono-cultures or co-cultures. Data presented as mean + SEM, n = 3 replicates. Image Credit: Sartorius
Summary and outlook
The quantification of biologically relevant secreted chemokines and cytokines is critical in fundamental research and drug development because it enables a better understanding of immune cell activation and complex cellular signaling cascades involved in neuroinflammation and neurodegeneration.
Existing systems for protein detection in solution often face trade-offs among ease of use, speed, multiplexing capability, and cost.
To efficiently screen secreted proteins, techniques must be fast and sensitive enough to enable large throughput while simultaneously being cost-effective and producing useful results.
The data presented demonstrates how iQue Qbeads® and iQue® HTS by Cytometry can be used to simultaneously explore a wide range of released proteins in physiologically relevant immune cell or iPSC-derived neuroinflammatory models.
These adaptable assays allow target proteins of interest to be investigated in a miniaturized format, allowing for the evaluation of various treatments at multiple timepoints and the observation of a wide range of biological reactions.
This study demonstrated how they can be used to gain a better understanding of temporal secreted protein release in response to pro-inflammatory stimuli, as well as to investigate phenotypic variations between healthy and diseased states.
References
- Liu, C., et al. (2021). Cytokines: from Clinical Significance to Quantification. Advanced Science, 8(15), p.2004433. DOI: 10.1002/advs.202004433. https://advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202004433.
- DiSabato, D.J., Quan, N. and Godbout, J.P. (2016). Neuroinflammation: the Devil Is in the Details. Journal of Neurochemistry, (online) 139(2), pp.136–153. DOI: 10.1111/jnc.13607. https://onlinelibrary.wiley.com/doi/10.1111/jnc.13607.
- Zhang, W., et al. (2023). Role of neuroinflammation in neurodegeneration development. Nature, (online) 8(1). DOI: 10.1038/s41392-023-01486-5. https://www.nature.com/articles/s41392-023-01486-5.
- Olivia, Ingibjörg Sigvaldadóttir and Eyer, K. (2021). Measuring single‐cell protein secretion in immunology: Technologies, advances, and applications. European Journal of Immunology, 51(6), pp.1334–1347. DOI: 10.1002/eji.202048976. https://onlinelibrary.wiley.com/doi/10.1002/eji.202048976.
- Leng, S.X., et al. (2008). ELISA and Multiplex Technologies for Cytokine Measurement in Inflammation and Aging Research. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, (online) 63(8), pp.879–884. DOI: 10.1093/gerona/63.8.879. https://academic.oup.com/biomedgerontology/article-abstract/63/8/879/567391?redirectedFrom=fulltext&login=false.
- bit.bio. (2026). Welcome To Zscaler Directory Authentication. (online) Available at: https://www.bit.bio/resources/user-manuals/microglia.
- AXOL Bioscience. (2024). Cortical Excitatory Neurons User Guide. (online) Available at: https://axolbio.com/publications/https-axolbio-com-wp-content-uploads-2024-06-axol-user-guide-monoculture-axocells-cortical-excitatory-neurons-version-2-june-2024-pdf/.
- AXOL Bioscience. (2025). Microglia User Guide. (online) Available at: https://axolbio.com/publications/microglia-user-guide/.
- Nam, H.Y., et al. (2018). Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. Journal of Neuroinflammation, 15(1). DOI: 10.1186/s12974-018-1308-0. https://link.springer.com/article/10.1186/s12974-018-1308-0.
- Ngabire, D., et al. (2018). Anti-Inflammatory Effects of Aster incisus through the Inhibition of NF-κB, MAPK, and Akt Pathways in LPS-Stimulated RAW 264.7 Macrophages. Mediators of Inflammation, 2018, pp.1–10. DOI: 10.1155/2018/4675204. https://onlinelibrary.wiley.com/doi/10.1155/2018/4675204.
- Xie, S., et al. (2014). Identification of a Role for the PI3K/AKT/mTOR Signaling Pathway in Innate Immune Cells. PLoS ONE, 9(4), p.e94496. DOI: 10.1371/journal.pone.0094496. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0094496.
- Chu, E., et al. (2021). Dysregulated phosphoinositide 3-kinase signaling in microglia: shaping chronic neuroinflammation. Journal of Neuroinflammation, 18(1). DOI: 10.1186/s12974-021-02325-6. https://link.springer.com/article/10.1186/s12974-021-02325-6.
- Manning, B.D. and Toker, A. (2017). AKT/PKB Signaling: Navigating the Network. Cell, 169(3), pp.381–405. DOI: 10.1016/j.cell.2017.04.001. https://linkinghub.elsevier.com/retrieve/pii/S0092867417304130.
- Genin, M., et al. (2015). M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer, 15(1). DOI: 10.1186/s12885-015-1546-9. https://link.springer.com/article/10.1186/s12885-015-1546-9.
- Mantovani, A., et al. (2002). Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology, 23(11), pp.549–555. DOI: 10.1016/s1471-4906(02)02302-5. https://www.cell.com/trends/immunology/abstract/S1471-4906(02)02302-5.
- Gordon, S. and Taylor, P.R. (2005). Monocyte and macrophage heterogeneity. Nature Reviews Immunology, 5(12), pp.953–964. DOI: 10.1038/nri1733. https://www.nature.com/articles/nri1733.
- Zhu, Z. and Huangfu, D. (2013). Human pluripotent stem cells: an emerging model in developmental biology. Development, (online)140(4), pp.705–717. DOI: 10.1242/dev.086165. https://journals.biologists.com/dev/article/140/4/705/76744/Human-pluripotent-stem-cells-an-emerging-model-in.
- Li, S., et al. (2022). Microglia subtypes show substrate- and time-dependent phagocytosis preferences and phenotype plasticity. Frontiers in Immunology, 13. DOI: 10.3389/fimmu.2022.945485. https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.945485/full.
- Woodburn, S.C., Bollinger, J.L., and Wohleb, E.S. (2021). The semantics of microglia activation: neuroinflammation, homeostasis, and stress. Journal of Neuroinflammation, (online) 18(1). DOI: 10.1186/s12974-021-02309-6. https://link.springer.com/article/10.1186/s12974-021-02309-6.
- Kwon, H.S. and Koh, S.-H. (2020). Neuroinflammation in Neurodegenerative disorders: the Roles of Microglia and Astrocytes. Translational Neurodegeneration, 9(1). DOI: 10.1186/s40035-020-00221-2. https://link.springer.com/article/10.1186/s40035-020-00221-2.
- Westin, K., et al. (2012). CCL2 Is Associated with a Faster Rate of Cognitive Decline during Early Stages of Alzheimer’s Disease. PLoS ONE, 7(1), p.e30525. DOI: 10.1371/journal.pone.0030525. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0030525.
About Sartorius
Sartorius accelerates the development of breakthrough therapeutics with innovative solutions for lab filtration, cell/protein analysis, and more. Visit sartorius.com/biologics to ensure confidence in your biotherapeutic discovery and development program.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.